- Biochemistry and Molecular Biology
- Biostatistics
- Environmental Health and Engineering
- Epidemiology
- Health Policy and Management
- Health, Behavior and Society
- International Health
- Mental Health
- Molecular Microbiology and Immunology
- Population, Family and Reproductive Health
- Program Finder
- Admissions Services
- Course Directory
- Academic Calendar
- Hybrid Campus
- Lecture Series
- Convocation
- Strategy and Development
- Implementation and Impact
- Integrity and Oversight
- In the School
- In the Field
- In Baltimore
- Resources for Practitioners
- Articles & News Releases
- In The News
- Statements & Announcements
- At a Glance
- Student Life
- Strategic Priorities
- Inclusion, Diversity, Anti-Racism, and Equity (IDARE)
- What is Public Health?

Understanding the CDC’s Updated COVID Isolation Guidance
The updated recommendations align guidance for COVID infection with that for other common respiratory viruses.
Aliza Rosen
For the first time since 2021, the Centers for Disease Control and Prevention has updated its COVID isolation guidance.
Specifically, it has shifted the recommendation that someone who tests positive for COVID isolate for five days to a timeline based on the progression of the person’s symptoms. The update is part of a larger strategy to provide one set of recommendations for most common respiratory illnesses , including COVID, influenza, and respiratory syncytial virus (RSV).
In this Q&A, virologist Andy Pekosz , PhD, a professor in Molecular Microbiology and Immunology , explains the CDC’s new isolation guidance, the reasons for the update, and why the prevention and treatment strategies we’ve all become accustomed to still play an important part in reducing respiratory virus transmission.
What are the updated recommendations for someone who comes down with a respiratory infection?
The updated guidance from the CDC is to “stay home and away from others (including people you live with who are not sick) if you have respiratory virus symptoms that aren't better explained by another cause.” You can resume normal activities once your symptoms are improving and you’ve been fever-free—without the aid of fever-reducing medications—for at least 24 hours.
For the five days after you resume your normal activities, you should take extra precautions, like wearing a well-fitting mask and maintaining distance from others, gathering outdoors or in well-ventilated areas, cleaning hands and high-touch surfaces often, and testing when possible before gathering with others. If symptoms or fever return, you should start back at square one: staying home and away from others until you’ve been improving and fever-free for at least 24 hours.
What should you do if you’re at higher risk of severe illness?
If you’re at higher risk of severe illness—generally, this is older adults and young children, pregnant people, people with disabilities, and people with compromised immune systems—seek testing and contact your physician. If you test positive for COVID or flu, there are antiviral medications that can be taken within a few days of symptom onset and are extremely effective in reducing the likelihood that your symptoms become severe or that you need to be hospitalized.
How does this differ from previous guidance?
Before this, the CDC recommended that people who test positive for COVID should isolate away from others for five days and wear a well-fitting mask around others for the following five days. This was different from the general guidance for other common respiratory viruses, like flu and RSV.
Now there is no one-size-fits-all duration for how long to isolate; rather, you can resume regular activities—ideally still using other prevention strategies, like masking and distancing—based on when your symptoms have improved and your fever has gone away.
This marks a significant change in guidance for people who test positive for COVID. Why has the guidance changed?
The CDC has simplified its recommendations for how long to stay home and isolate after testing positive or experiencing symptoms to be consistent across COVID-19, influenza, and RSV infections. This way, anyone who develops symptoms can follow the same isolation guidance, irrespective of what respiratory virus they’re infected with.
It’s important to note, though, that this guidance on how long to isolate is just one part of a larger strategy for combating respiratory viruses that includes:
- Being up to date on recommended vaccines.
- Practicing good hygiene regarding hand-washing, sneezing, and coughing.
- Being aware of antiviral treatment options for COVID-19 and influenza.
- Taking steps to improve indoor air quality.
If the guidance is the same for all respiratory viruses, is it still important to test to know what someone is sick with?
Yes, testing is still needed in order to get a prescription for antivirals to treat COVID-19 or influenza. Those antivirals have been shown to reduce disease severity in several different groups, so if you are in a high risk group, be sure to test early and contact your physician so you can get the antiviral prescriptions as soon as possible.
Testing can also play an important role in preventing transmission, particularly if you were recently around someone who has since become sick, or if you plan to spend time with someone who is at higher risk of severe infection.
For COVID in particular, rapid home antigen tests are a great way to determine whether you’re still infectious and able to infect others. Symptom severity can be fairly subjective and a presence or lack of symptoms does not always align with infectiousness , so testing out of isolation for COVID is still good practice if you have access to tests.
Does this new guidance mean that all of these respiratory viruses pose the same risk?
No, COVID-19 is still causing more cases and more severe disease than influenza or RSV. A person’s risk for severe infection will also vary based on a number of factors, including age and health conditions .
The updated guidance acknowledges that we can simplify the recommendations for what to do after becoming infected with a respiratory virus, as part of the larger strategy to address spread.
The CDC also recently recommended that people over age 65 receive an additional dose of this year’s COVID vaccine . What drove that decision?
There are a few reasons behind this new recommendation for older adults . First, most severe COVID infections are occurring in individuals 65 years and older who have not been vaccinated recently. The CDC’s recommendation notes that more than half of COVID hospitalizations between October 2023 and December 2023 occurred in adults over 65.
Second, we know immunity after vaccination wanes over a few months, so an additional dose will provide renewed protection through the spring. New COVID variants like JN.1 that are circulating now have some mutations that improve their ability to evade vaccine-induced immunity, but the antibodies made through vaccination still recognize them. It’s not a perfect match, but a second dose of this year’s vaccine will provide protection against current variants to an age group at increased risk of severe illness, hospitalization, and death.
When should people over 65 get this additional dose of the current COVID vaccine?
The recommendation from the CDC is for people 65 and older who have already received one dose of the 2023-24 COVID vaccine to get a second shot at least four months after their most recent dose .
For people in that age group who haven’t had the 2023-24 vaccine, there’s no need to wait. They can get their shot now to be protected through the spring.
Will there be an updated COVID-19 vaccine for these newer variants?
We can likely expect to see a new COVID-19 vaccine available this fall, just like we see new, updated influenza vaccines each fall. This spring—typically around May—a decision will be made on which variants the updated vaccine will be designed around, and like we saw in 2023, the new vaccine will be available in the fall as we head into the typical respiratory virus season.
Aliza Rosen is a digital content strategist in the Office of External Affairs at the Johns Hopkins Bloomberg School of Public Health.
- More Americans Could Benefit from Paxlovid for COVID Infection
- What to Know About the Updated COVID-19 Vaccine for Fall/Winter 2023
Related Content

A Forgotten, Yet Life-Threatening Infection

What to Know About COVID FLiRT Variants

Rotavirus the Leading Cause of Diarrheal Deaths Among Children Under 5, New Analysis Finds

Outbreak Preparedness for All

Peter Agre’s Third Act
Are You Still Contagious With COVID After 5 Days? Here's What We Know
During a facebook live last month, chicago department of public health commissioner dr. allison arwady reported that recent studies have shown the incubation period for covid has dropped to three days with recent variants, published september 9, 2022 • updated on september 9, 2022 at 2:14 pm.
As COVID's incubation period changes, what does that mean for isolation time and how long you are contagious?
During a Facebook Live last month, Chicago Department of Public Health Commissioner Dr. Allison Arwady reported that recent studies have shown the incubation period for COVID has dropped to three days with recent variants.
Watch NBC 5 Chicago news stream free, 24/7, wherever you are
"So if you go back to like alpha variant, beta, delta - early on, it was about a five-day incubation period on average. So, if you were exposed to COVID, on average we were seeing people take about five days for someone to end up testing positive - and remember that went from four to five, out to 10, out to 14," she said. "The reason ... we only use 10 days now, it is because that timing has shortened. And so, more recently with BA.4, BA.5, that's all the way down to about three days now. So on average, people are testing positive about three days after, but you can have someone positive up to 10 days."
Arwady said most elements of COVID are moving faster with the BA.4 and BA.5 subvariants.
"That is excellent news from a control perspective and because one of the biggest challenges of COVID is that... when the incubation period is long, you can get infected with COVID, potentially have a long time before you have significant symptoms and it'll be spreading it," she said. "So it's good news to see that incubation period getting shorter."
According to the Centers for Disease Control and Prevention, someone with COVID-19 is "considered infectious starting two days before they develop symptoms, or two days before the date of their positive test if they do not have symptoms."
Regardless of symptoms, those who test positive are advised to take specific precautions for at least 10 days, though five days is the new isolation minimum, per the CDC. But does that mean you aren't contagious after five days?

6 children among 7 hospitalized after crash on Near South Side

More Chicagoans speak out against iFLIP Chicago following initial NBC 5 Responds report
The answer depends.
People are likely the most infectious in the first five days after contracting the virus, health officials state, hence why isolation is recommended.
Feeling out of the loop? We'll catch you up on the Chicago news you need to know. Sign up for the weekly Chicago Catch-Up newsletter here.
As a precaution, those who test positive are encouraged to wear a well-fitting face mask through day 10, even though the risk has gone down at that point.
Once day 10 rolls around, the risk drops significantly, including for those who have lingering symptoms, Arwady previously stated.
"If you're mostly feeling well, especially if what is still kind of lingering is a cough or a little bit of cough tends to be the last thing to go away after any virus, it is unlikely that you are still spreading disease," the doctor said.
If after five days you are fever-free for 24 hours without the use of medication, and your symptoms are improving, or you never had symptoms, you may end isolation.
But if you're still getting a positive test after six to 10 days, Arwady said you could still be contagious.
"Generally if their symptoms have resolved, they are very unlikely to still be spreading a lot of COVID. But if you still have a positive rapid test, make sure you are wearing a mask, consider isolating," Arwady said last month.
She clarified that the positive test applies more so to rapid results, as opposed to PCR. PCR COVID tests can stay positive for a "very long time" after recovering from the virus because they pick up on any dead infection.
Explaining most Americans have some level of immunity, the CDC issued major changes to its COVID guidelines in August , altering the recommendations for quarantine, social distancing and even testing.
Here's a breakdown of what we know:
When do you need to isolate?
According to the CDC, regardless of vaccination status, you should isolate from others when you have COVID-19. You should also isolate if you are sick or suspect that you have COVID-19 but are waiting on test results.
It's important to note that if you were exposed to COVID-19, the Food and Drug Administration now recommends you take three home tests instead of two to make sure you’re not infected.
The new guidance applies to people without symptoms who think they may have been exposed.
Previously, the FDA had advised taking two rapid antigen tests over two or three days to rule out infection. But the agency says new studies suggest that protocol can miss too many infections, and could result in people spreading the coronavirus to others, especially if they don't develop symptoms.
How long should you isolate?
If you test positive for COVID-19, the guidance states that you should stay home for at least five days and isolate from others in your home. You are likely most infectious during these first five days.
When you end isolation, you should still avoid being around people who are most at-risk until at least day 11.
After you have ended isolation, you'll also need to wear a mask through day 10, per the guidelines. The CDC also notes, however, that if you have access to antigen tests, "you should consider using them."
"With two sequential negative tests 48 hours apart, you may remove your mask sooner than day 10," the guidance states, adding that if your antigen test results are positive, "you may still be infectious."
Those who continue to test positive should continue masking.
"You should continue wearing a mask and wait at least 48 hours before taking another test," the CDC recommends. "Continue taking antigen tests at least 48 hours apart until you have two sequential negative results. This may mean you need to continue wearing a mask and testing beyond day 10."
If your symptoms worsen or return after you end isolation, you'll need to restart your isolation at day 0, per the guidelines.
How do you calculate isolation time?
The CDC states that isolation for those who have COVID is counted in days, but it depends on if you have symptoms.
If you have no symptoms :
- Day 0 is the day you were tested (not the day you received your positive test result)
- Day 1 is the first full day following the day you were tested
- If you develop symptoms within 10 days of when you were tested, the clock restarts at day 0 on the day of symptom onset
If you have symptoms :
- Day 0 of isolation is the day of symptom onset, regardless of when you tested positive
- Day 1 is the first full day after the day your symptoms started
What does isolation include?
- Wear a high-quality mask if you must be around others at home and in public.
- Do not go places where you are unable to wear a mask.
- Do not travel.
- Stay home and separate from others as much as possible.
- Use a separate bathroom, if possible.
- Take steps to improve ventilation at home, if possible.
- Don’t share personal household items, like cups, towels, and utensils.
- Monitor your symptoms . If you have an emergency warning sign (like trouble breathing), seek emergency medical care immediately.
What do you need to do to end isolation?
If you had no symptoms, you can end isolation after day 5, according to the CDC.
If you had symptoms, however, you can only end isolation after day 5 if:
- You are fever-free for 24 hours (without the use of fever-reducing medication)
- Your symptoms are improving
If you still have a fever or your other symptoms have not improved, continue to isolate until they improve, the guidelines state.
How severe your symptoms are can also play a role.
If you had moderate illness - such as shortness of breath or difficulty breathing - or severe illness, including hospitalization due to COVID-19, or if you have a weakened immune system, you need to isolate through day 10.
If you had severe illness or have a weakened immune system, you'll want to consult your doctor before ending isolation as you may need a viral test to do so.
Do you need to quarantine?
The CDC previously said that if people who are not up to date on their COVID-19 vaccinations come into close contact with a person who tests positive, they should stay home for at least five days. Now the agency says quarantining at home is not necessary, but it urges those people to wear a high-quality mask for 10 days and get tested after five.
This article tagged under:
- COVID-19 travel advice
Considering travel during the pandemic? Take precautions to protect yourself from COVID-19.
A coronavirus disease 2019 (COVID-19) vaccine can prevent you from getting COVID-19 or from becoming seriously ill due to COVID-19 . But even if you're vaccinated, it's still a good idea to take precautions to protect yourself and others while traveling during the COVID-19 pandemic.
If you've had all recommended COVID-19 vaccine doses, including boosters, you're less likely to become seriously ill or spread COVID-19 . You can then travel more safely within the U.S. and internationally. But international travel can still increase your risk of getting new COVID-19 variants.
The Centers for Disease Control and Prevention (CDC) recommends that you should avoid travel until you've had all recommended COVID-19 vaccine and booster doses.
Before you travel
As you think about making travel plans, consider these questions:
- Have you been vaccinated against COVID-19 ? If you haven't, get vaccinated. If the vaccine requires two doses, wait two weeks after getting your second vaccine dose to travel. If the vaccine requires one dose, wait two weeks after getting the vaccine to travel. It takes time for your body to build protection after any vaccination.
- Have you had any booster doses? Having all recommended COVID-19 vaccine doses, including boosters, increases your protection from serious illness.
- Are you at increased risk for severe illness? Anyone can get COVID-19 . But older adults and people of any age with certain medical conditions are at increased risk for severe illness from COVID-19 .
- Do you live with someone who's at increased risk for severe illness? If you get infected while traveling, you can spread the COVID-19 virus to the people you live with when you return, even if you don't have symptoms.
- Does your home or destination have requirements or restrictions for travelers? Even if you've had all recommended vaccine doses, you must follow local, state and federal testing and travel rules.
Check local requirements, restrictions and situations
Some state, local and territorial governments have requirements, such as requiring people to wear masks, get tested, be vaccinated or stay isolated for a period of time after arrival. Before you go, check for requirements at your destination and anywhere you might stop along the way.
Keep in mind these can change often and quickly depending on local conditions. It's also important to understand that the COVID-19 situation, such as the level of spread and presence of variants, varies in each country. Check back for updates as your trip gets closer.
Travel and testing
For vaccinated people.
If you have been fully vaccinated, the CDC states that you don't need to get tested before or after your trip within the U.S. or stay home (quarantine) after you return.
If you're planning to travel internationally outside the U.S., the CDC states you don't need to get tested before your trip unless it's required at your destination. Before arriving to the U.S., you need a negative test within the last day before your arrival or a record of recovery from COVID-19 in the last three months.
After you arrive in the U.S., the CDC recommends getting tested with a viral test 3 to 5 days after your trip. If you're traveling to the U.S. and you aren't a citizen, you need to be fully vaccinated and have proof of vaccination.
You don't need to quarantine when you arrive in the U.S. But check for any symptoms. Stay at home if you develop symptoms.
For unvaccinated people
Testing before and after travel can lower the risk of spreading the virus that causes COVID-19 . If you haven't been vaccinated, the CDC recommends getting a viral test within three days before your trip. Delay travel if you're waiting for test results. Keep a copy of your results with you when you travel.
Repeat the test 3 to 5 days after your trip. Stay home for five days after travel.
If at any point you test positive for the virus that causes COVID-19 , stay home. Stay at home and away from others if you develop symptoms. Follow public health recommendations.
Stay safe when you travel
In the U.S., you must wear a face mask on planes, buses, trains and other forms of public transportation. The mask must fit snugly and cover both your mouth and nose.
Follow these steps to protect yourself and others when you travel:
- Get vaccinated.
- Keep distance between yourself and others (within about 6 feet, or 2 meters) when you're in indoor public spaces if you're not fully vaccinated. This is especially important if you have a higher risk of serious illness.
- Avoid contact with anyone who is sick or has symptoms.
- Avoid crowds and indoor places that have poor air flow (ventilation).
- Don't touch frequently touched surfaces, such as handrails, elevator buttons and kiosks. If you must touch these surfaces, use hand sanitizer or wash your hands afterward.
- Wear a face mask in indoor public spaces. The CDC recommends wearing the most protective mask possible that you'll wear regularly and that fits. If you are in an area with a high number of new COVID-19 cases, wear a mask in indoor public places and outdoors in crowded areas or when you're in close contact with people who aren't vaccinated.
- Avoid touching your eyes, nose and mouth.
- Cover coughs and sneezes.
- Wash your hands often with soap and water for at least 20 seconds.
- If soap and water aren't available, use a hand sanitizer that contains at least 60% alcohol. Cover all surfaces of your hands and rub your hands together until they feel dry.
- Don't eat or drink on public transportation. That way you can keep your mask on the whole time.
Because of the high air flow and air filter efficiency on airplanes, most viruses such as the COVID-19 virus don't spread easily on flights. Wearing masks on planes has likely helped lower the risk of getting the COVID-19 virus on flights too.
However, air travel involves spending time in security lines and airport terminals, which can bring you in close contact with other people. Getting vaccinated and wearing a mask when traveling can help protect you from COVID-19 while traveling.
The Transportation Security Administration (TSA) has increased cleaning and disinfecting of surfaces and equipment, including bins, at screening checkpoints. TSA has also made changes to the screening process:
- Travelers must wear masks during screening. However, TSA employees may ask travelers to adjust masks for identification purposes.
- Travelers should keep a distance of 6 feet apart from other travelers when possible.
- Instead of handing boarding passes to TSA officers, travelers should place passes (paper or electronic) directly on the scanner and then hold them up for inspection.
- Each traveler may have one container of hand sanitizer up to 12 ounces (about 350 milliliters) in a carry-on bag. These containers will need to be taken out for screening.
- Personal items such as keys, wallets and phones should be placed in carry-on bags instead of bins. This reduces the handling of these items during screening.
- Food items should be carried in a plastic bag and placed in a bin for screening. Separating food from carry-on bags lessens the likelihood that screeners will need to open bags for inspection.
Be sure to wash your hands with soap and water for at least 20 seconds directly before and after going through screening.
Public transportation
If you travel by bus or train and you aren't vaccinated, be aware that sitting or standing within 6 feet (2 meters) of others for a long period can put you at higher risk of getting or spreading COVID-19 . Follow the precautions described above for protecting yourself during travel.
Even if you fly, you may need transportation once you arrive at your destination. You can search car rental options and their cleaning policies on the internet. If you plan to stay at a hotel, check into shuttle service availability.
If you'll be using public transportation and you aren't vaccinated, continue physical distancing and wearing a mask after reaching your destination.
Hotels and other lodging
The hotel industry knows that travelers are concerned about COVID-19 and safety. Check any major hotel's website for information about how it's protecting guests and staff. Some best practices include:
- Enhanced cleaning procedures
- Physical distancing recommendations indoors for people who aren't vaccinated
- Mask-wearing and regular hand-washing by staff
- Mask-wearing indoors for guests in public places in areas that have high cases of COVID-19
- Vaccine recommendations for staff
- Isolation and testing guidelines for staff who've been exposed to COVID-19
- Contactless payment
- Set of rules in case a guest becomes ill, such as closing the room for cleaning and disinfecting
- Indoor air quality measures, such as regular system and air filter maintenance, and suggestions to add air cleaners that can filter viruses and bacteria from the air
Vacation rentals, too, are enhancing their cleaning procedures. They're committed to following public health guidelines, such as using masks and gloves when cleaning, and building in a waiting period between guests.
Make a packing list
When it's time to pack for your trip, grab any medications you may need on your trip and these essential safe-travel supplies:
- Alcohol-based hand sanitizer (at least 60% alcohol)
- Disinfectant wipes (at least 70% alcohol)
- Thermometer
Considerations for people at increased risk
Anyone can get very ill from the virus that causes COVID-19 . But older adults and people of any age with certain medical conditions are at increased risk for severe illness. This may include people with cancer, serious heart problems and a weakened immune system. Getting the recommended COVID-19 vaccine and booster doses can help lower your risk of being severely ill from COVID-19 .
Travel increases your chance of getting and spreading COVID-19 . If you're unvaccinated, staying home is the best way to protect yourself and others from COVID-19 . If you must travel and aren't vaccinated, talk with your health care provider and ask about any additional precautions you may need to take.
Remember safety first
Even the most detailed and organized plans may need to be set aside when someone gets ill. Stay home if you or any of your travel companions:
- Have signs or symptoms, are sick or think you have COVID-19
- Are waiting for results of a COVID-19 test
- Have been diagnosed with COVID-19
- Have had close contact with someone with COVID-19 in the past five days and you're not up to date with your COVID-19 vaccines
If you've had close contact with someone with COVID-19 , get tested after at least five days. Wait to travel until you have a negative test. Wear a mask if you travel up to 10 days after you've had close contact with someone with COVID-19 .
- How to protect yourself and others. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html. Accessed Feb. 4, 2022.
- Domestic travel during COVID-19. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/travelers/travel-during-covid19.html. Accessed Feb. 4, 2022.
- Requirement for face masks on public transportation conveyances and at transportation hubs. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/travelers/face-masks-public-transportation.html. Accessed Feb. 4, 2022.
- International travel. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/travelers/international-travel/index.html. Accessed Feb. 4, 2022.
- U.S citizens, U.S. nationals, U.S. lawful permanent residents, and immigrants: Travel to and from the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/travelers/international-travel-during-covid19.html. Accessed Feb. 4, 2022.
- Non-US. citizen, non-U.S. immigrants: Air travel to the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/travelers/noncitizens-US-air-travel.html. Accessed Feb. 4, 2022.
- People with certain medical conditions. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed Feb. 4, 2022.
- Stay up to date with your vaccines. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html. Accessed Feb. 4, 2022.
- Pack smart. Centers for Disease Control and Prevention. https://wwwnc.cdc.gov/travel/page/pack-smart. Accessed Feb. 4, 2022.
- Travel: Frequently asked questions. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/travelers/faqs.html. Accessed Feb. 7, 2022.
- Coronavirus (COVID-19) information. Transportation Security Administration. https://www.tsa.gov/coronavirus. Accessed Feb. 7, 2022.
- WHO advice for international traffic in relation to the SARS-CoV-2 Omicron variant (B.1.1.529). World Health Organization. https://www.who.int/news-room/articles-detail/who-advice-for-international-traffic-in-relation-to-the-sars-cov-2-omicron-variant. Accessed Feb. 7, 2022.
- VRHP/VRMA Cleaning guidelines for COVID-19. Vacation Rental Management Association. https://www.vrma.org/page/vrhp/vrma-cleaning-guidelines-for-covid-19. Accessed Feb. 7, 2022.
- Safe stay. American Hotel & Lodging Association. https://www.ahla.com/safestay. Accessed Feb. 7, 2022.
- Khatib AN, et al. COVID-19 transmission and the safety of air travel during the pandemic: A scoping review. Current Opinion in Infectious Diseases. 2021; doi:10.1097/QCO.0000000000000771.
Products and Services
- A Book: Endemic - A Post-Pandemic Playbook
- Begin Exploring Women's Health Solutions at Mayo Clinic Store
- A Book: Future Care
- Antibiotics: Are you misusing them?
- COVID-19 and vitamin D
- Convalescent plasma therapy
- Coronavirus disease 2019 (COVID-19)
- COVID-19: How can I protect myself?
- Herd immunity and respiratory illness
- COVID-19 and pets
- COVID-19 and your mental health
- COVID-19 antibody testing
- COVID-19, cold, allergies and the flu
- Long-term effects of COVID-19
- COVID-19 tests
- COVID-19 drugs: Are there any that work?
- COVID-19 in babies and children
- Coronavirus infection by race
- COVID-19 vaccine: Should I reschedule my mammogram?
- COVID-19 vaccines for kids: What you need to know
- COVID-19 vaccines
- COVID-19 variant
- COVID-19 vs. flu: Similarities and differences
- COVID-19: Who's at higher risk of serious symptoms?
- Debunking coronavirus myths
- Different COVID-19 vaccines
- Extracorporeal membrane oxygenation (ECMO)
- Fever: First aid
- Fever treatment: Quick guide to treating a fever
- Fight coronavirus (COVID-19) transmission at home
- Honey: An effective cough remedy?
- How do COVID-19 antibody tests differ from diagnostic tests?
- How to measure your respiratory rate
- How to take your pulse
- How to take your temperature
- How well do face masks protect against COVID-19?
- Is hydroxychloroquine a treatment for COVID-19?
- Loss of smell
- Mayo Clinic Minute: You're washing your hands all wrong
- Mayo Clinic Minute: How dirty are common surfaces?
- Multisystem inflammatory syndrome in children (MIS-C)
- Nausea and vomiting
- Pregnancy and COVID-19
- Safe outdoor activities during the COVID-19 pandemic
- Safety tips for attending school during COVID-19
- Sex and COVID-19
- Shortness of breath
- Thermometers: Understand the options
- Treating COVID-19 at home
- Unusual symptoms of coronavirus
- Vaccine guidance from Mayo Clinic
- Watery eyes
U.S. travel resources
- Check CDC recommendations for travel within the U.S.
- Review testing requirements for travel to the U.S.
- Look up restrictions at your destination .
- Review airport security measures .
Related resources
We’re transforming healthcare.
Make a gift now and help create new and better solutions for more than 1.3 million patients who turn to Mayo Clinic each year.

Explaining the New CDC Guidance on What To Do if You Have COVID-19
By Kate Yandell
Posted on March 15, 2024
Q: Is one day isolation sufficient to stop forward transmission of COVID-19?
A: People with COVID-19 could potentially transmit it to others well beyond a day after developing symptoms or testing positive. New guidance from the CDC advises people to isolate until they have been fever-free and with symptoms improving for at least 24 hours, and then take precautions for five days, which covers the period when “most people are still infectious.”
FULL ANSWER
The Centers for Disease Control and Prevention on March 1 updated its guidance on preventing the spread of respiratory viruses, consolidating advice on a range of common respiratory illnesses including COVID-19, flu and respiratory syncytial virus, or RSV.
Since December 2021 , the agency had recommended individuals isolate for at least five days after developing symptoms of COVID-19, or after a positive test if asymptomatic. After five days, the agency recommended various symptom-based criteria for leaving isolation combined with additional continued precautions, such as masking.

The new guidance drops the standard minimum of five days of isolation in favor of a symptom-based approach. The agency advises people to stay home and away from others when they are sick with a respiratory virus. People can cease isolation if, over a period of 24 hours, their overall symptoms have been improving and they have been fever-free without using fever-reducing medications.
Many people have had questions about what the new guidance means for people who have COVID-19. Some, like our reader, have referred to the idea that the guidance means only one day of isolation is needed. “do you agree with Biden that one day isolation for covid is fine and dandy??” asked one person on X, formerly known as Twitter.
But that’s not what Biden or the CDC is recommending.
“It’s not saying isolate for 24 hours,” epidemiologist Ronit Dalmat , a research scientist at the University of Washington, told us, referring to the CDC guidance. “It’s saying if you have a fever, absolutely stay home” until it has been gone for 24 hours, and also stay home until other symptoms are improving.
Nor does the CDC say people are guaranteed not to spread COVID-19 or other respiratory illnesses after their symptoms have improved. “Keep in mind that you may still be able to spread the virus that made you sick, even if you are feeling better,” the guidance says. “You are likely to be less contagious at this time, depending on factors like how long you were sick or how sick you were.”
The guidance recommends continuing to take precautions for five days after resuming normal activities. These include physical distancing, testing, improving air quality, using good hygiene and wearing a well-fitting mask, such as an N95 or KN95.
“The total number of days of precautions when sick, that is, a period of staying home and away from others plus 5 days of additional actions, covers the period during which most people are still infectious,” the CDC wrote in an FAQ.
“That whole period could be quite a while,” Dalmat said. “That could be 10 days for some people.”
The CDC said in background materials accompanying the new guidance that it looked at data from countries and states that had adopted similar policies for COVID-19 isolation and had not seen “clear increases in community transmission or hospitalization rates.”
“The updated guidance on steps to prevent spread when you are sick particularly reflects the key reality that many people with respiratory virus symptoms do not know the specific virus they are infected with,” the CDC said. The agency noted that its survey data indicated less than half of people with cold or cough symptoms would take an at-home COVID-19 test.
Some on social media have misinterpreted the guidance as an admission that it was always reasonable to liken COVID-19 to the flu, as was done early in the pandemic despite the marked difference in the diseases’ severity.
But the new CDC guidance acknowledges the continued seriousness of COVID-19 while also detailing the ways in which treatments, vaccines and population immunity have improved outcomes for people with the disease.
“COVID-19 remains a greater cause of severe illness and death than other respiratory viruses, but the differences between these rates are much smaller than they were earlier in the pandemic,” the CDC said . The agency explained that the risks are reduced due to the availability of COVID-19 treatments and population immunity to the virus, both from vaccination and prior infection. The agency also said that long COVID remains a risk, although the prevalence appears to be falling.
The Science on COVID-19 Transmission
Whether someone transmits COVID-19 depends on multiple factors . These include a person’s infectious viral load, but also the susceptibility of the people the infected person encounters and the precautions taken.
There’s no one-size-fits-all answer to how long a particular individual will shed infectious virus and how much they will shed. “Everybody has a slightly different ability to control the amount of virus in their system, which is a part of what makes the virus shed,” Dalmat said. Variation in how people’s bodies fight a virus affects “how much virus you are putting in the world that is infectious.”
There’s evidence that a relatively small number of people who shed particularly high levels of the virus over the course of their infections have been responsible for a disproportionate number of COVID-19 cases, and many people with COVID-19 do not infect others.
However, according to the CDC, the data on the typical overall length of shedding has not significantly changed, even as new variants of SARS-CoV-2 — the virus that causes COVID-19 — have arisen. “Even as the SARS-CoV-2 virus has continued to evolve, the duration of shedding infectious virus has remained relatively consistent, with most individuals no longer infectious after 8-10 days,” the agency said .
The CDC accompanied this statement with a figure showing data collected by the Respiratory Virus Transmission Network from five U.S. sites between November 2022 and May 2023 (see below). One line on the graph (light blue) shows how often researchers were able to isolate and grow — or culture — virus from people with COVID-19.
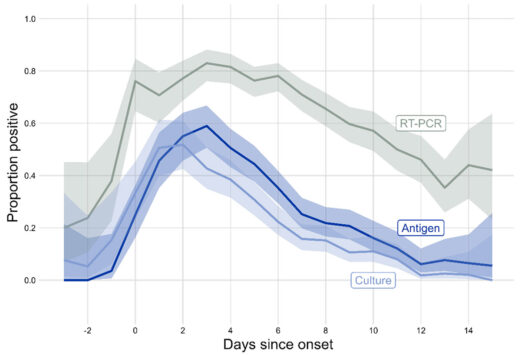
Trying to culture the virus that causes COVID-19 from a respiratory sample — a laborious process used in research — indicates whether someone is carrying infectious virus. The figure shows that the proportion of people with culturable virus began to increase two days before symptoms begin, or before a positive test for those who were asymptomatic, peaking around one to two days after symptom onset. After that, the rate began falling, with around one-third of people having culturable virus at day five. By day 10, the percentage had dropped to around 10%.
A different study , published in 2023 in the International Journal of Infectious Diseases, combined data from multiple studies done in people diagnosed with COVID-19 in 2021 and 2022. The average duration of shedding of culturable virus was just over five days from symptom onset or first positive PCR test, whichever came first.
Another metric for assessing infectiousness in people with COVID-19 is viral load, often measured as the amount of viral materials, such as RNA or proteins, found in a respiratory sample. A 2023 study published in Clinical Infectious Diseases found that median viral load for people diagnosed with COVID-19 peaked around three or four days after symptoms started. The study assessed people seeking testing for respiratory infections between April 2022 and April 2023.

Someone who is shedding infectious virus may or may not transmit it to others. One factor is that the average person is less susceptible to infection today than they were early in the pandemic, Dalmat said.
“Even if the person is producing the exact same amount of virus today as they could have three years ago, the people on the other end on average are less likely to get infected,” Dalmat said, explaining that today more than 98% of the population has had some exposure to COVID-19 itself, COVID-19 vaccines or both.
When people do get infected, the cases tend to be less severe. “Among the people who get infected with COVID these days, on average it is much rarer that it turns into a very serious illness,” Dalmat said, while also acknowledging that a lot of individuals “are still very vulnerable.” People at elevated risk for severe disease include those who are elderly or immune compromised.
While the CDC guidance harmonizes suggested precautions for COVID-19 and other common respiratory viruses, there are differences in the details of how COVID-19 and other respiratory viruses are spread.
The new guidance is meant to be a general rule of thumb but does not apply to health care settings or cases where there is an outbreak of a disease that requires special instructions, the CDC said. The CDC also said the agency is working on specific guidance for schools, which should be available prior to the 2024/2025 school year.
Masks, Tests and Other Precautions
Isolating from other people when sick is a key way to reduce one’s risk of spreading COVID-19. But the CDC guidance lists additional ways to reduce the chances of spreading a respiratory illness.
Masks can help prevent the wearer from spreading a respiratory virus. They can also protect others from inhaling a virus, particularly well-fitting masks such as N95 or KN95 respirators, the guidance says. Individuals can take measures to improve their hygiene and the air quality in their surroundings and maintain physical distance from others, such as by avoiding crowded spaces.
The CDC still recommends testing to help high-risk people who are sick determine whether to seek treatment for a specific virus. For instance, someone with COVID-19 may benefit from receiving Paxlovid within five days of when their symptoms start. The guidance also lists tests as a tool that can help people decide when they need to take precautions to avoid spreading disease.

At-home rapid antigen tests can be helpful for people who are recovering from COVID-19 and want to see if they still have infectious virus, Dalmat said. In their research, she and her colleagues found that among people who tested positive for COVID-19 on a rapid antigen test, subsequent negative antigen test results were “very, very highly correlated to whether you had infectious virus or not,” she said. That means people with COVID-19 who start to test negative on rapid antigen tests as they get better likely are no longer at risk of infecting others.
However, the CDC cautions that rapid antigen tests early in the course of a person’s infection often miss COVID-19. People who are sick should be taking precautions regardless of test results, Dalmat said. “They shouldn’t test and have a negative test be the end of it,” she said.
The authors of the Clinical Infectious Diseases study , which measured viral loads over the course of infection, wrote that “our data in combination with others’ suggest that symptomatic individuals testing positive for SARS-CoV-2 by PCR currently may not reliably test positive on a rapid antigen test until the third, fourth, or even fifth day of symptoms.”
The CDC guidance says people can end isolation when they have been fever-free and their symptoms have been improving for at least 24 hours. Dalmat cautioned that the definition of improving symptoms is somewhat ambiguous.
“Symptoms improving can mean different things to different people,” Dalmat said, adding that people should make sure their symptoms are truly getting better. “If your symptoms are not really improving – not kind of plateauing but really improving — you should continue to stay home and continue to take whatever measures you are taking in your household.”
Editor’s note: SciCheck’s articles providing accurate health information and correcting health misinformation are made possible by a grant from the Robert Wood Johnson Foundation. The foundation has no control over FactCheck.org’s editorial decisions, and the views expressed in our articles do not necessarily reflect the views of the foundation.
Branswell, Helen. “ CDC Eases Isolation Guidance for Covid and Other Respiratory Illnesses .” STAT. 1 Mar 2024.
“ Preventing Spread of Respiratory Viruses When You’re Sick .” CDC website. Updated 1 Mar 2024.
“ CDC’s Updated Respiratory Virus Guidance: What to Do When You Are Sick .” CDC website. 1 Mar 2024.
“ CDC Updates and Shortens Recommended Isolation and Quarantine Period for General Population .” CDC website. 27 Dec 2021.
“ Isolation and Precautions for People with COVID-19 .” CDC website. Updated 11 Mar 2023.
Cali Dreaming NaphiSoc (@NaphiSoc). “ Prof Hotez: do you agree with Biden that one day isolation for covid is fine and dandy?? ” X. 2 Mar 2024.
Dalmat, Ronit. Interview with FactCheck.org.
“ Respiratory Virus Guidance Update FAQs .” CDC website. Updated 1 Mar 2024.
“ Background for CDC’s Updated Respiratory Virus Guidance .” CDC website. Updated 1 Mar 2024.
Matt Kim 🇰🇷🇺🇸 (@mattattack009). “ Zero Accountability .” Instagram. 4 Mar 2024.
DiedSuddenly (@DiedSuddenly_). “ Turns out everything they told you about Covid was a lie. Of course they knew this 3 years ago, and they’ll show zero remorse for what they have done .” X. 2 Mar 2024.
Citizen Free Press (@CitizenFreePres). “ … and then one day, four years later on a Friday afternoon when no one was looking, the CDC admitted that the great conspiracy theory about Covid was true .” X. 1 Mar 2024.
Rieder, Rem. “ Trump’s Deceptive Comparison of the Coronavirus to the Flu .” FactCheck.org. 9 Sep 2020.
“ How is COVID-19 transmitted? ” FactCheck.org. Updated 11 Feb 2022.
Puhach, Olha et al. “ SARS-CoV-2 Viral Load and Shedding Kinetics .” Nature Reviews Microbiology. 2 Dec 2022.
Wu, Yu et al. “ Duration of Viable Virus Shedding and Polymerase Chain Reaction Positivity of the SARS-CoV-2 Omicron Variant in the Upper Respiratory Tract: A Systematic Review and Meta-Analysis .” International Journal of Infectious Diseases. 18 Feb 2023.
Frediani, Jennifer K. et al. “ The New Normal: Delayed Peak SARS-CoV-2 Viral Loads Relative to Symptom Onset and Implications for COVID-19 Testing Programs .” Clinical Infectious Diseases. 28 Sep 2023.
Cevik, Muge and Kalil, Andre C. “ Omicron Variant: Assessing the Duration of Viral Shedding and Its Implications .” Clinical Microbiology and Infection. 25 Nov 2022.
“ Risk Factors for Severe Illness from Respiratory Viruses .” CDC website. Updated 1 Mar 2024.
Wu, Katherine J. “ Why Are We Still Flu-Ifying COVID? ” The Atlantic. 28 Feb 2024.
“ Masks and Respiratory Viruses Prevention .” CDC website. Updated 1 Mar 2024.
“ Hygiene and Respiratory Viruses Prevention .” CDC website. Updated 1 Mar 2024.
“ Taking Steps for Cleaner Air for Respiratory Virus Prevention .” CDC website. Updated 1 Mar 2024.
“ About Physical Distancing and Respiratory Viruses .” CDC website. Updated 1 Mar 2024.
“ Preventing Respiratory Viruses .” CDC website. Updated 1 Mar 2024.
“ COVID-19 Treatments and Medications .” CDC website. Updated 15 Mar 2024.
“ Testing and Respiratory Viruses .” CDC website. Updated 1 Mar 2024.
Drain, Paul K. et al. “ Duration of Viral Infectiousness and Correlation with Symptoms and Diagnostic Testing in Non-Hospitalized Adults during Acute SARS-CoV-2 Infection: A Longitudinal Cohort Study .” Journal of Clinical Virology. 3 Mar 2023.
Get Daily Travel Tips & Deals!
By proceeding, you agree to our Privacy Policy and Terms of Use .
When Can I Travel After Testing Positive for COVID-19?
Caroline Morse Teel
Caroline Morse Teel is the Executive Editor for SmarterTravel Media. Caroline has a passion for adventure travel and has hiked to the top of Mt. Kilimanjaro and the bottom of the Grand Canyon in pursuit of a good story. Follow her around the world on Instagram @TravelWithCaroline .
Travel Smarter! Sign up for our free newsletter.
With preflight COVID-19 tests required to visit many destinations (and to return to the United States), there’s a chance you could get a positive result before your next flight. If that happens, the first question on your mind will be, “When can I travel after testing positive for COVID?”
The answer will depend on three things: the country you’re currently in, your destination country, and the airline you’re flying.
When Can You Fly Back to the United States After Testing Positive for COVID?
If you’re flying back to the United States, the Centers for Disease Control and Prevention (CDC) tells people who have tested positive for COVID, “Do not travel until a full 10 days after your symptoms started or the date your positive test was taken if you had no symptoms.”
Although the CDC changed the recommended isolation period from 10 days to five days, the agency still advises people not to travel for 10 days after testing positive/symptoms starting.
Jasmine Reed, a spokesperson for the CDC, explains “The travel guidance considers the higher risk of getting and spreading COVID-19 associated with travel. Travel is a door-to-door experience that results in close contact with others, often for prolonged periods in crowded confined spaces.”
Fortunately, after you’ve tested positive you will not need a negative COVID test to reenter the U.S. You can instead travel with a letter of recovery from your doctor, along with proof of a positive test taken within 90 days.
According to the CDC, you can use a letter of recovery only if “you have met the criteria to travel” which includes completing a 10-day quarantine.
The First At-Home Molecular COVID Test: Cue COVID-19 Test Review
Check With Your Airline
The most important COVID requirement to check before you fly is with your airline. Airlines have different rules regarding how soon passengers will be allowed to fly after testing positive for COVID. Certain international airlines insist on a longer delay of up to 14 days, whereas others allow for a shorter quarantine. Look for a written policy on your airline’s website, or call to clarify before rebooking your ticket.
When Can You Fly Internationally After Testing Positive for COVID?

This depends on your destination. Some countries accept a proof of recovery and a positive COVID test (similar to the United States), whereas others will only accept a negative test. Check with the State Department’s website for the country you’re visiting for the most up-to-date information.
You Might Also Like:
We hand-pick everything we recommend and select items through testing and reviews. Some products are sent to us free of charge with no incentive to offer a favorable review. We offer our unbiased opinions and do not accept compensation to review products. All items are in stock and prices are accurate at the time of publication. If you buy something through our links, we may earn a commission.
Top Fares From

Don't see a fare you like? View all flight deals from your city.
Today's top travel deals.
Brought to you by ShermansTravel
Southwest Ireland: 8-Night Trip, Incl. Guinness...
Specialized Travel Services

Luxe, 7-Night Caribbean & Mexico Cruise...
Regent Seven Seas Cruises

Ohio: Daily Car Rentals from Cincinnati

Trending on SmarterTravel
You are using an outdated browser. Upgrade your browser today or install Google Chrome Frame to better experience this site.
What is COVID-19?
Who can get covid-19, can i travel if i recently had covid-19, what can travelers do to prevent covid-19, more information.
CDC Respiratory Virus Guidance has been updated. The content of this page will be updated soon.
Coronavirus disease 2019 (COVID-19) is a respiratory illness caused by the virus SARS-CoV-2. The virus spreads mainly from person to person through respiratory droplets and small particles produced when an infected person coughs, sneezes, or talks. The virus spreads easily in crowded or poorly ventilated indoor settings.
People with COVID-19 have reported a wide range of symptoms – ranging from no or mild symptoms to severe illness. Symptoms may appear 2–14 days after exposure to the virus. Possible symptoms include fever, chills, cough, shortness of breath, fatigue, muscle aches, headache, new loss of taste and smell, sore throat, runny nose, nausea, vomiting, or diarrhea.
Anyone can get COVID-19. However, some people are more likely than others to get very sick if they get COVID-19. These include people who are older, are immunocompromised , or have certain disabilities , or have underlying health conditions . Vaccination, past infection, and timely access to testing and treatment can help protect you from getting very sick from COVID-19.
Yes, you can travel once you have ended isolation . Check CDC guidance for additional precautions, including testing and wearing a mask around others. If you recently had COVID-19 and are recommended to wear a mask, do not travel on public transportation such as airplanes, buses, and trains if you are unable to wear a mask whenever around others.
Get up to date with your COVID-19 vaccines before you travel and take steps to protect yourself and others . Consider wearing a mask in crowded or poorly ventilated indoor areas, including on public transportation and in transportation hubs. Take additional precautions if you were recently exposed to a person with COVID-19. Don’t travel while sick.
If you have a weakened immune system or are at increased risk for severe disease talk to a healthcare professional before you decide to travel. If you travel, take multiple prevention steps to provide additional layers of protection from COVID-19, even if you are up to date with your COVID-19 vaccines. These include improving ventilation and spending more time outdoors, avoiding sick people, getting tested for COVID-19 if you develop symptoms, staying home if you have or think you have COVID-19, and seeking treatment if you have COVID-19.
Consider getting travel insurance in case you need medical care abroad .
Consider getting a COVID-19 test if you:
- Develop COVID-19 symptoms before, during, or after travel.
- Will be traveling to visit someone who is at higher risk of getting very sick from COVID-19.
- Were in a situation with a greater risk of exposure during travel (e.g., in an indoor, crowded space like an airport terminal while not wearing a mask).
If you traveled and feel sick, particularly if you have a fever, talk to a healthcare professional, and tell them about your recent travel.
- Masking During Travel
File Formats Help:
- Adobe PDF file
- Microsoft PowerPoint file
- Microsoft Word file
- Microsoft Excel file
- Audio/Video file
- Apple Quicktime file
- RealPlayer file
- Zip Archive file
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
National Geographic content straight to your inbox—sign up for our popular newsletters here

Should you still travel if you have Covid?
With rules relaxed, it can be hard to know what to do if you test positive before a trip. Does catching the virus still spell the end for your plans?
Compulsory PCR tests, face masks, vaccination certificates — at the height of the pandemic, travel meant navigating reams of red tape and checking a long list of requirements before you’d even set foot on a plane. Now the rules have been relaxed, travellers are largely responsible for making their own decisions should they test positive. From the legal requirements to the moral debate, here’s what you need to know.
What’s the official advice?
In the UK, there’s no legal requirement to self-isolate if you test positive for the virus, and current NHS advice for adults is to ‘try to stay at home and avoid contact with other people for five days’. So travelling with Covid is permitted — but you have to accept that you risk passing the virus to others.
Which countries still impose restrictions?
Europe has scrapped all Covid entry rules, but it’s worth noting that some countries in the rest of the world still don’t let you travel freely. Tourist destinations such as the Philippines, Bolivia and China still have entry requirements in place; for example, the latter insists that visitors take a lateral flow/rapid antigen test at least 48 hours before boarding a flight, among other restrictions. While many operators, including airlines, have removed the requirement to wear a mask while travelling, some countries including China insist on it in some circumstances.
To avoid unnecessary surprises on arrival, consult the Foreign, Commonwealth & Development Office’s (FCDO) travel advice pages for each country that you’re planning to visit or travel through. Take note of the entry requirements section, which will show whether the destination currently has any Covid-specific rules or restrictions in place.
Should I still cancel my trip if I test positive?
Now that travellers are largely no longer legally obliged to take a test or disclose the result, it’s important to make an informed decision. No one wants to miss out on a planned trip, but virologist Stephen Griffin encourages people to “prioritise the most vulnerable people in our society”. According to the Office for National Statistics, the risk of death involving Covid remains significantly greater for the immunocompromised — on your next flight, for example, you could be sitting next to someone who’s more vulnerable because they’ve just finished chemotherapy. The guilt of potentially infecting other travellers could be enough to cast a shadow over any getaway.
How easy is it to change your travel plans?
Often, it’s not very easy at all. Most operators have scrapped cancellation policies introduced during the pandemic, and are well within their rights to tell you to take the trip or forfeit your rights if you test positive.Travel writer Lottie Gross recently found herself wrangling with a campsite for a refund after notifying the owners she’d tested positive and being asked by them to stay away. “I don’t entirely regret my decision to inform the campsite of my Covid infection,” she says, “but it was a little frustrating to be told I couldn’t go and that I also couldn’t have a refund.”
If this happens, there may still be options open to you. “You could claim on your travel insurance if your policy covers it and you’re able to provide evidence of your positive test,” says Confused.com’s lifestyle insurance expert Matthew Harwood. “This will vary depending on the provider and their specific terms and conditions, so always double-check what you’ll be covered for before buying a policy.”
It’s also worth checking the small print in your travel booking, as your terms and conditions could legally compel you to divulge test results to your tour operator, accommodation provider or airline.
What precautions should I take if I still want to travel?
If you test positive ahead of a trip and want to minimise the risk of spreading the infection, Professor Griffin advises taking “every precaution to reduce interactions with other people”. He says: “Stay outside (on a ferry deck, for example) or in well-ventilated spaces if possible, and wear a well-fitted, filtering respirator mask, ideally an FFP3, unless distanced from others.”
Introducing Nat Geo Kids Book Bundle!
Related topics.
- PUBLIC HEALTH
- EDUCATIONAL TRAVEL
- CORONAVIRUS
You May Also Like

This airline is now weighing passengers — but why?

The new rules on carrying liquids through UK airport security

The essential guide to visiting Texas

An insider's guide to Denver, Colorado's wildly creative capital

10 reasons to visit the East Coast in 2024
- Environment
- Paid Content
History & Culture
- History & Culture
- History Magazine
- Mind, Body, Wonder
- Destination Guide
- Terms of Use
- Privacy Policy
- Your US State Privacy Rights
- Children's Online Privacy Policy
- Interest-Based Ads
- About Nielsen Measurement
- Do Not Sell or Share My Personal Information
- Nat Geo Home
- Attend a Live Event
- Book a Trip
- Inspire Your Kids
- Shop Nat Geo
- Visit the D.C. Museum
- Learn About Our Impact
- Support Our Mission
- Advertise With Us
- Customer Service
- Renew Subscription
- Manage Your Subscription
- Work at Nat Geo
- Sign Up for Our Newsletters
- Contribute to Protect the Planet
Copyright © 1996-2015 National Geographic Society Copyright © 2015-2024 National Geographic Partners, LLC. All rights reserved
How soon can you travel after testing positive for COVID-19?

A positive COVID-19 test during a trip can throw all your travel plans into limbo . But even a positive test in the weeks before you travel can be cause for concern.
If you're wondering when you'll be cleared to travel again after testing positive for COVID-19 , it's an important question: Here's everything you need to know.
For more TPG news delivered each morning to your inbox, sign up for our free daily newsletter .
What are the rules on flying back to the US?

If you've traveled internationally in the past year, you're likely familiar with the rules to fly back to the United States, but they've changed several times .
All travelers coming to the U.S., vaccinated or not, must produce a negative COVID-19 test taken within one calendar day of their departure. (The previous policy allowed vaccinated international travelers to show a negative test taken within three days before departure.) Additionally, international foreign travelers can enter the U.S. with proof of vaccination and a negative COVID-19 test .
If you have a positive test, does that mean you're banned from flying back to the U.S.? Not quite — but the rules can be a little confusing even if you're a seasoned traveler. If you recently had COVID-19 but recovered from the virus, you can still travel back to the U.S., but you'll need the proper paperwork. Let's break it down.
What paperwork do I need to travel after testing positive?

People who recovered from COVID-19 may continue to test positive for the virus up to three months after infection, even after they've recovered.
According to the U.S. Centers for Disease Control and Prevention, travelers who recently recovered from COVID-19 can fly back to the U.S. with proof that they've recovered from COVID-19 instead of a negative test. This proof can include your positive COVID-19 viral test result, but it has to be taken no more than 90 days before your flight's departure from a foreign country.
Additionally, you will also need a signed letter from a licensed healthcare professional stating that you're cleared to travel back to the U.S. According to the CDC, the letter from a healthcare provider must include:
- Information that identifies you personally (such as your name and date of birth) and matches your passport.
- The letter must be signed and dated by the healthcare provider.
- The letter must be on official letterhead that contains the name, address and phone number of the healthcare provider or public health official who signed the letter.
The CDC says the positive test result and letter together are referred to as "documentation of recovery." If you tested positive, that's the only way to be able to fly back to the U.S. if you've recovered from the virus but don't have a negative test result.
Proof of recovery is also acceptable for certain destinations, so you may be able to use this documentation to travel abroad after you're cleared by a doctor to travel.
What if I can't show proof of recovery?
The CDC is pretty strict on this rule: You either need a negative COVID-19 test or proof of recovery. The agency says, "If you have recovered from COVID-19 but are not able to obtain documentation of recovery that fulfills the requirements, you will need to show a negative COVID-19 viral test result from a sample taken no more than one day before your flight to the US departs."
How soon can I leave my destination after a positive COVID-19 test?

Travelers have to consider the possibility of testing positive for COVID-19 while abroad. What happens after the positive test depends entirely on the destination and, in some cases, your vaccination and booster status.
For instance, if you test positive while in France , you'll have to quarantine for seven days if you're fully vaccinated with a booster dose. However, you can leave quarantine after five days with a negative antigen or RT-PCR test result and if you've had no symptoms in 48 hours. If you are not fully vaccinated (France will soon require travelers to have a booster to be considered fully vaccinated ) or not vaccinated and test positive, you must isolate for 10 days, though the quarantine can be shortened.
So if you have international travel planned, you'll need to read up on the rules around how long you'll have to stay in quarantine and the documentation you'll need for your flight back to the U.S.
clock This article was published more than 2 years ago
What 4 health experts say about travel after covid-19 recovery
You’ve recovered from the omicron variant. Can you travel like it’s 2019?

When I got the coronavirus in January, I spent the better part of two weeks in bed, too tired to do much. As I recovered slowly, a thought kept churning in my head as I considered my future immunity: “What does this mean for travel?”
The Centers for Disease Control and Prevention advises people not to “travel until a full 10 days after your symptoms started or the date your positive test was taken if you had no symptoms.” In the 90 days after you’ve fully recovered and meet criteria to end isolation, the CDC says , you can travel safely. If you’re not fully vaccinated, delay travel until you are, or incorporate testing into your trip plans if you must travel.
But health experts said life after infection comes with caveats, especially as we learn more about omicron. Here’s what four experts advise.
6 questions about travel after recovering from covid, answered
‘There’s a wide range of behaviors that are acceptable’
Céline Gounder, an infectious-disease expert at New York University and a member of President Biden’s covid-19 transition task force, says based on the rate she has seen antibodies decline after infection, “you probably do have at least a couple of months of some kind of protection against both infection and disease.”
The protection doesn’t work like a switch, and it depends on whether a person has had the coronavirus before and whether they are vaccinated.
“It’s sort of this steady decline,” Gounder says. “Somewhere between three and six months, you certainly would be at risk for reinfection.”
How you approach travel after a coronavirus infection will depend on your demographic and risk factors. While travel will never be 100 percent safe, “I think as long as you’re not putting others in danger, and you’re not being reckless to the point of really adding to the burden on health-care systems … there’s a wide range of behaviors that are acceptable,” Gounder says.
She finds trips where you can spend most time outdoors to be the least risky, including going camping and visiting destinations where you can eat outside at restaurants. If you are flying anywhere, Gounder recommends wearing an N95 mask, like the 3M Aura one (“They’re actually pretty comfortable,” she says) and keeping it on as much as possible from the time you leave your house to the time you arrive at your final destination.
How 3 travelers with disabilities or chronic illness navigate the world
‘We’re never going to go back to the way it was’
People should remain vigilant about coronavirus mitigation efforts, even if they’ve recently had it, says Brian C. Castrucci, the president and chief executive of de Beaumont Foundation, a public health charity.
For vaccinated and boosted travelers who have had omicron, “you probably do have immunity, but we don’t know for how long,” Castrucci says. “The immunity is not going to be enduring, and it’s still possible to get a severe infection that has ongoing symptoms.”
Just as there are still safety protocols in place at airports following the 9/11 attacks, we can expect coronavirus protocols to stay put, Castrucci says.
“We’re never going to go back to the way it was,” he says. “Even if this becomes endemic, it’s going to then indelibly change how we go about our lives.”
Castrucci says what that looks like for travelers going forward is wearing a well-fitting mask in public places, knowing the vaccination and case rate of the place you’re visiting, taking a coronavirus test before you leave, and packing rapid tests in case you feel sick on the road.
What to know about cruise travel while omicron spreads
‘Natural infection wanes a lot faster than vaccination’
If you’re vaccinated, boosted and recently recovered from the coronavirus, “your chances of having a serious reinfection are not very high,” says Karl E. Minges, the interim dean of the University of New Haven’s School of Health Sciences in Connecticut.
However, “you’re not protected forever,” Minges says. “Natural infection wanes a lot faster than vaccination. So if you have been infected by omicron and you’re unvaccinated, do get vaccinated.”
Recovering from infection “doesn’t change the calculus about what activities are safer as compared to others,” Minges says, encouraging recovered people to follow the same precautions they would before. For example, take a rapid test before doing something on the upper level of your risk tolerance, like traveling.
‘We saw many people infected with delta who got reinfected with omicron’
Jayne Morgan, a cardiologist and executive director of the covid-19 task force at Piedmont Healthcare, does not want recently recovered travelers to have a false sense of security, because the future of mutating variants is impossible to predict.
“You should still exercise caution because you still have the ability to be reinfected with new variants that could come about,” Morgan says. “We saw many people infected with delta who got reinfected with omicron.”
While Morgan says 90 days is usually how long immunity lasts before it starts to drop, there is inconsistency with how it drops. With much unknown about the omicron variant, it is unclear how long natural immunity lasts and whether it will be effective in protecting against future variants.
Beyond keeping up with covid-cautious behavior as a social responsibility to vulnerable people around you, Morgan says not to let up your defense because of the state of the pandemic.
“We are still in the middle of a pandemic with exceptionally high numbers,” she says. “We are in a worse situation with [case] numbers now than we were with our first three surges.”
That doesn’t mean you can’t take a vacation. Morgan says she advocates for trips with outdoor activities, which she recognizes may be difficult, but not impossible, to pull off in cold winter weather.
“This is a great time to take a ski trip and be outdoors,” she says.
Coronavirus: What you need to know
Covid isolation guidelines: Americans who test positive for the coronavirus no longer need to routinely stay home from work and school for five days under new guidance planned by the Centers for Disease Control and Prevention. The change has raised concerns among medically vulnerable people .
New coronavirus variant: The CDC said it is monitoring a variant called KP.2 and does not see evidence it causes more severe illness than other strains. It also identified a second emergent variant, KP.1.1. But it is KP.2 that is leading the pack. Both new variants belong to a group of coronavirus variants dubbed “FLiRT” by scientists.
Latest coronavirus booster: The CDC recommends that anyone 6 months or older gets an updated coronavirus shot , but the vaccine rollout has seen some hiccups , especially for children . Here’s what you need to know about the latest coronavirus vaccines , including when you should get it.

- Skip to main content
- Keyboard shortcuts for audio player

Coronavirus Updates
Cdc says travel is safe for fully vaccinated people, but opposes nonessential trips.
Rachel Treisman

The Centers for Disease Control and Prevention updated its domestic travel guidance for fully vaccinated people on Friday, lifting certain requirements while continuing to advise mitigation measures like mask-wearing and hand-washing. Angus Mordant/Bloomberg via Getty Images hide caption
The Centers for Disease Control and Prevention updated its domestic travel guidance for fully vaccinated people on Friday, lifting certain requirements while continuing to advise mitigation measures like mask-wearing and hand-washing.
The Centers for Disease Control and Prevention has updated its domestic travel guidance for fully vaccinated people, lifting certain testing and self-quarantine requirements and recommending precautions like wearing a mask and avoiding crowds. But health officials continue to discourage nonessential travel, citing a sustained rise in cases and hospitalizations.
The CDC updated its website on Friday to reflect the latest scientific evidence, writing that "people who are fully vaccinated with an FDA-authorized vaccine can travel safely within the United States."
The announcement comes less than a month after the CDC first released updated guidance about gatherings for fully vaccinated people, which it described as a "first step" toward returning to everyday activities.
Air Travel Is Opening Up Again, But That Doesn't Mean The Pandemic Is Over
The CDC considers someone fully vaccinated two weeks after they receive the last dose of vaccine. Those individuals will no longer need to get tested before or after travel unless their destination requires it, and do not need to self-quarantine upon return.
The new guidance means, for example, that fully vaccinated grandparents can fly to visit their healthy grandkids without getting a COVID-19 test or self-quarantining as long as they follow other recommended measures while traveling, according to CDC Director Rochelle Walensky.
Those measures include wearing a mask over their nose and mouth, staying 6 feet from others and washing their hands frequently. Masks are required on all planes traveling into, within or out of the U.S., under an executive order issued by President Biden.
But Walensky, speaking at a White House COVID-19 Response Team briefing on Friday, nonetheless discouraged all nonessential travel, citing a continued increase in the seven-day average of cases and hospitalizations.
"While we believe that fully vaccinated people can travel at low risk to themselves, CDC is not recommending travel at this time due to the rising number of cases," Walensky said.

CDC Director Fears 'Impending Doom' If U.S. Opens Too Quickly
She said that while vaccinated people can do more things safely, most Americans are not yet fully vaccinated. Those who are not must have a negative test 1-3 days before they travel under CDC guidance. They must either get tested 3-5 days after they return and self-quarantine for 7 days, or self-quarantine for 10 days with no test.
Walensky said on Monday that there is more travel occurring now than throughout the pandemic, including the winter holidays. She acknowledged that people have been looking to get away over spring break or take advantage of what they perceive as a "relative paucity in cases," and she said the country was seeing an uptick in cases as a result.
"The thing that's different this time is that we actually have it in our power to be done with the scale of the vaccination," she said. "And that will be so much slower if we have another surge to deal with as well."
The U.S. is already seeing an uptick in domestic travel, and many Americans are looking to book trips in the coming months in what experts described to NPR as a sign of "clear pent up demand for travel."
As the country's supply of COVID-19 doses has grown, so has Biden's goal for the number of shots in arms during his first 100 days, doubling the target to 200 million by the end of this month. Many states have already expanded eligibility to all adults or are set to do so in the coming weeks, well ahead of the president's May 1 deadline.
According to NPR's vaccine tracker , 16.9% of the U.S. population is fully vaccinated, and 30% has had at least one dose. Researchers estimate that 70% to 85% of the country would need to have immunity for COVID-19 to stop spreading through communities.
International travel restrictions remain
The CDC is not lifting travel restrictions barring the entry of most non-U.S. citizens from places including China, Brazil, South Africa and parts of Europe. It will continue to require airline passengers entering the U.S. to get a test within three days of their departure and show proof of a negative result before boarding.
The travel industry has been pushing for some of these restrictions to end. A group of 26 organizations sent a letter to White House COVID-19 czar Jeffrey Zients urging the federal government "to partner with us to develop, by May 1, 2021, a risk-based, data-driven roadmap to rescind inbound international travel restrictions."

While Some Spring Breakers Swarm Beaches, Many Stay Home, Dreaming Of Summer Travel
"To be clear, at this time, we do not support removal or easing of core public health protections, such as the universal mask mandate, inbound international testing requirement, physical distancing or other measures that have made travel safer and reduced transmission of the virus," they wrote. "However, the data and science demonstrate that the right public health measures are now in place to effectively mitigate risk and allow for the safe removal of entry restrictions."
Travel and tourism have taken a considerable hit because of the pandemic with industry groups noting that overseas travel to the U.S. declined by 81% in 2020, causing billions of dollars in losses. Without lifting international travel bans, the U.S. Travel Association estimates that some 1.1 million American jobs will not be restored and billions in spending will be lost by the end of the year.
"Fortunately, enough progress has been made on the health front that a rebound for domestic leisure travel looks possible this year, but that alone won't get the job done," Roger Dow, the association's president and CEO, said in a statement . "A full travel recovery will depend on reopening international markets, and we must also contend with the challenge of reviving business travel."

Fauci Expects Surge In Vaccinations To Keep A 4th Coronavirus Wave At Bay
- Centers for Disease Control and Prevention
- COVID-19 vaccine
CDC updates Covid isolation guidelines for people who test positive

People who test positive for Covid no longer need to isolate for five days , the Centers for Disease Control and Prevention said Friday.
The CDC’s new guidance now matches public health advice for flu and other respiratory illnesses: Stay home when you’re sick, but return to school or work once you’re feeling better and you’ve been without a fever for 24 hours.
The shift reflects sustained decreases in the most severe outcomes of Covid since the beginning of the pandemic, as well as a recognition that many people aren’t testing themselves for Covid anyway.
“Folks often don’t know what virus they have when they first get sick, so this will help them know what to do, regardless,” CDC director Dr. Mandy Cohen said during a media briefing Friday.
Over the past couple of years, weekly hospital admissions for Covid have fallen by more than 75%, and deaths have decreased by more than 90%, Cohen said.
“To put that differently, in 2021, Covid was the third leading cause of death in the United States. Last year, it was the 10th,” Dr. Brendan Jackson, head of respiratory virus response within the CDC’s National Center for Immunization and Respiratory Diseases, said during the briefing.
Many doctors have been urging the CDC to lift isolation guidance for months, saying it did little to stop the spread of Covid.
The experiences of California and Oregon , which previously lifted their Covid isolation guidelines, proved that to be true.
“Recent data indicate that California and Oregon, where isolation guidance looks more like CDC’s updated recommendations, are not experiencing higher Covid-19 emergency department visits or hospitalizations,” Jackson said.
Changing the Covid isolation to mirror what’s recommended for flu and other respiratory illnesses makes sense to Dr. David Margolius, the public health director for the city of Cleveland.
“We’ve gotten to the point where we are suffering from flu at a higher rate than Covid,” he said. “What this guidance will do is help to reinforce that— regardless of what contagious respiratory viral infection you have — stay home when you’re sick, come back when you’re better.”
Dr. Kristin Englund, an infectious diseases expert at the Cleveland Clinic, said the new guidance would be beneficial in curbing the spread of all respiratory viruses.
“I think this is going to help us in the coming years to make sure that our numbers of influenza and RSV cases can also be cut down, not just Covid,” she said.
Latest news on Covid
- Common Covid symptoms follow a pattern now, doctors say.
- Covid during pregnancy can cause health issues in babies.
- How big of a risk is coinfection with Covid and other viruses?
Still, the decision was likely to draw criticism from some clinicians who point to the fact that the U.S. logged 17,310 new Covid hospitalizations in the past week alone.
“It’s something that is likely to draw a wide array of opinions and perhaps even conflicting opinions,” said Dr. Faisal Khan, Seattle’s director of public health. “But [the CDC’s] rationale is sound in that the pandemic is now in a very different phase from where it was in 2021 or 2022 or 2023.”
Though the isolation guidelines have been wiped away, the CDC still encourages people to play it safe for five days after they are feeling better. That includes masking around vulnerable people and opening windows to improve the flow of fresh air indoors.
The majority of viral spread happens when people are the sickest. “As the days go on, less virus spreads,” Cohen said.
People at higher risk for severe Covid complications, such as the elderly, people with weak immune systems and pregnant women, may need to take additional precautions.
Dr. Katie Passaretti, chief epidemiologist at Atrium Health in Charlotte, said it was a “move in the positive direction.”
“We are continuing to edge into what the world looks like after Covid, with Covid being one of many respiratory viruses that are certain that circulate,” she said.
The new guidance is for the general public only, and does not include isolation guidelines in hospital settings, which is generally 10 days.
On Wednesday, the agency said that adults 65 and older should get a booster shot of the Covid vaccine this spring. It’s anticipated that the nation will experience an uptick in the illness later this summer.
Winter and summer waves of Covid have emerged over the past four years, with cases peaking in January and August, respectively, according to the CDC .
Another, reformulated, shot is expected to be available and recommended this fall.
CDC’s main tips for reducing Covid spread:
- Get the Covid vaccine whenever it is available. Cohen said that 95% of people who were hospitalized with Covid this past winter had not received the latest vaccine.
- Cover coughs and sneezes, and wash hands frequently.
- Increase ventilation by opening windows, using air purifiers and gathering outside when possible.
Erika Edwards is a health and medical news writer and reporter for NBC News and "TODAY."

Sign up for the Health News Florida newsletter
Here's how to decide if you're safe to go out when you're recovering from omicron.

The good news is, the numbers of COVID-19 cases are plummeting across the country. But there's still a stunning amount of virus spreading in many places, with more than 100,000 reported cases a day. If you have a current infection, you might be wondering, just how long am I infectious? And when is it safe for me to step out and socialize again without risking getting others sick?
The answer depends on whom you ask.
According to guidance from the Centers for Disease Control and Prevention, you can exit isolation five days after a positive test or the start of symptoms, so long as your symptoms are improving and you keep wearing a mask around others an additional five days.
However, this guidance comes with caveats: It's largely based on data from prior variants, and it was shaped by practical considerations – namely, how to make sure workplaces had enough staff to keep functioning at a time when omicron infections were racing across the country.
Meanwhile, emerging science suggests that, with the omicron variant, as many as half of the people infected will still be potentially infectious on day five – and some may be for a few days beyond.
"What we know based on the data so far is we can't reliably use five days as a way to exit isolation," says Dr. Peter Chin-Hong , a professor of medicine and infectious disease specialist at the University of California, San Francisco.
So how can you know if you are ready to rejoin the world — without potentially infecting others? Here are some science-based criteria that can help you figure it out.
How much time has passed?
At least three studies have found that people infected with omicron still have virus levels high enough to be contagious more than five days after their symptoms began.
Researchers looked at data from the National Basketball Association's extensive COVID testing program. Their study found that around 50% of people infected with omicron were still testing positive on a PCR test on day five. And compared with people infected with delta, there was a lot more variability in how long it took people with omicron to hit their peak viral load.
"For some people with omicron, it happens very, very fast. They turn positive and then they hit their peak very quickly. For others, it takes many days" – up to eight or even 10 days after turning positive, says the study's senior author, Dr. Yonatan Grad , an associate professor of immunology and infectious diseases at the Harvard T.H. Chan School of Public Health.
Another small study from Japan found that virus levels were highest on days three through six, and then gradually started to drop off. After 10 days, nobody in the study had infectious virus detectable on a PCR test.
And a third study, of 260 vaccinated health care workers in Chicago, found that overall, 43% were testing positive on rapid antigen tests five to 10 days after infection with omicron – even though they felt well enough to return to work. The rates of positivity "were higher on day six and seven and lower on days nine and 10," says study co-author Dr. Emily Landon of the University of Chicago Medicine.
Given all these findings so far, Landon says if you can't test again to exit isolation, waiting "eight days is a lot safer than six days." Just keep wearing your mask though day 10, she says.
After 10 days, you can consider yourself good to go, says Chin-Hong. He says multiple studies have shown that "there's very little, if any, transmission after day 10, regardless of the variant." There's one notable exception to this rule of thumb: If you're immunocompromised, you should wait 20 days to exit isolation, because research prior to omicron has shown that these patients tend to shed virus longer.
What symptoms do I still have, if any?
With prior variants, data suggested that people were most infectious just before and in the day after their symptoms developed, says Chin-Hong. That influenced the CDC's five-day guideline for exiting isolation.
But the relationship between symptoms and infectivity appears to have changed with omicron, according to Landon's research – in part because so many people are now vaccinated. Vaccination primes the immune system which means that "it doesn't take so long to rev up, so that it can react to even small amounts of virus in your body," Landon says. So symptoms can be a sort of early-warning siren of an infection, even if you're not yet contagious.
On the flipside, you can still be infectious even if you're feeling better. In Landon's study, health care workers were still testing positive even though they felt well enough to return to work. "We were really surprised," says Landon. "They didn't seem sick" – yet many still had virus levels likely to be infectious.
And you can continue to experience symptoms even after you are no longer infectious "because your immune system is still active," says Chin-Hong.
So how should symptoms guide you? First of all, if you've got a fever, that's a red flag, says Chin-Hong – "because the type of immune response you get that results in a fever is usually because there's a ton of virus floating around."
If you're fever-free but you are still feeling sick, "that's not a great sign" – keep isolating for as much of that 10 day period as possible, and keep masking around others, says Landon.
But if you are feeling better and 10 days have passed since the start of your symptoms or your first positive test, consider yourself good to go. One caveat: If you are planning to see someone vulnerable, it's best to wait until your symptoms have all resolved.
Can I use testing to decide when to come out of isolation?
First, make sure you understand which kind of test you are taking. A PCR test is very accurate at the start of an infection because it can detect and amplify even trace amounts of virus DNA. But a PCR is not the right choice to figure out when you are no longer infectious, because of its sensitivity, Grad explains.
"There are some people who have little blips of being PCR positive for weeks, or in some cases even months, after an infection" – even though they're no longer contagious, Grad says.
A better bet is to use a rapid antigen test, because they're "positive when your viral load is high," corresponding to levels when people are likely to be infectious, says Landon. So if you're negative on a rapid test and you don't have any symptoms, consider yourself in the clear, says Chin-Hong.
What if 10 days have passed and you're still testing positive on a rapid test? "That definitely happens, and we don't have a good answer" as to why, says Landon. One thing to look at is how faint the positive line is on the rapid test, she says, because research has shown that the darker or more intense the line is and the more quickly it shows up, the more virus is present in your nose. So if you're past day 10, you feel better and you're not immunocompromised, and the rapid test line "isn't very dark or it's taking longer to turn positive each day, you're probably safe to be out in the world," she says.
But if you're going to be around vulnerable people, such as someone who's had a recent organ transplant, is elderly or is otherwise at risk of severe disease with COVID, then even a very faint positive line should make you think hard about being in contact with these folks, Landon says.
And remember, when you're out in public, you never know when someone you interact with in the grocery store could be immunocompromised or highly vulnerable. So if you have any doubt of your infectiousness, wear a high-quality mask in public out of consideration for those people.
Copyright 2022 NPR. To see more, visit https://www.npr.org.

- COVID Questions
I Was Exposed to COVID-19. How Long Will It Take for Symptoms to Start?

Y ou get the dreaded text: the friend you just met for lunch tested positive for COVID-19 . Now you’re left to wonder if you, too, will get sick in the coming days.
But when should you expect symptoms to start if you do get sick? The answer has changed from the earliest days of COVID-19, experts say.
“In the beginning of the pandemic, we were really looking at seven to 10 days as the window of time where people had to quarantine or isolate after an exposure,” says Andrew Pekosz, a virologist at Johns Hopkins University. “That has shortened significantly now.”
How long does it take to develop COVID-19 symptoms?
An incubation period is the length of time it takes someone to develop symptoms after exposure to a pathogen. The incubation period for SARS-CoV-2, the virus that causes COVID-19, has shortened considerably since the virus first began circulating, recent data suggest. Incubation periods averaged about five days when the Alpha variant was dominant, about 4.5 days when Beta and Delta were dominant, and about 3.4 days once Omicron took over, according to a 2022 research review .
Newer research from various countries, including Japan , France , and Singapore , also suggests Omicron strains have incubation periods of about three days, or even a little less.
The virus' incubation period is likely shrinking for a few reasons, says Shane Crotty, chief scientific officer at the La Jolla Institute for Immunology. The virus has evolved over time, becoming faster and more adept at infecting humans, Crotty says. Nearly everyone has also now had at least one brush with COVID-19 , whether through vaccination or illness. Each encounter leaves behind instructions for the immune system, helping it recognize the virus faster the next time it appears.
“You having symptoms is all about your immune system being activated,” Crotty explains. “The whole pre-symptomatic period is bad news because your immune system has not managed to pull the fire alarm yet.” A shorter incubation period means that your body is “recognizing the virus faster and pulling those sprinkler systems faster.”
More From TIME
When should i test for covid-19 after an exposure, and when am i in the clear.
Federal health authorities, including the U.S. Centers for Disease Control and Prevention, recommend testing no sooner than five days after a COVID-19 exposure, unless you develop symptoms earlier. But since current variants seem to have incubation periods of around three days, Pekosz says it's appropriate to test as soon as day three, again unless symptoms start earlier.
Dr. Peter Hotez, dean of the National School of Tropical Medicine at Baylor College of Medicine, says he starts to feel more confident he's dodged an infection if he’s still feeling healthy three days after a potential exposure. But, "remember, incubation periods are statistical probabilities,” he says. “There’s always going to be outliers.” You could develop a sore throat or runny nose only a couple days after exposure to the virus, or you might not feel sick until day five—or, if you’re lucky, you may not get infected at all.
The timing of symptom onset depends on lots of factors, including the amount of virus to which someone was exposed, Hotez says. Their level of pre-existing immunity may also affect the likelihood or timing of getting sick, Crotty adds.
Given all this variation, Pekosz recommends monitoring your health for up to a week after an exposure and wearing a mask around other people during that time. Remember, too, that false negatives are possible on at-home tests . If you get a negative result, the U.S. Food and Drug Administration recommends taking at least one more test 48 hours later to confirm it.
What is the incubation period of JN.1?
It’s too soon to know exactly, but Hotez says JN.1 is likely to have an incubation period similar to that of other Omicron strains. One 2023 study found that while incubation periods have gotten shorter over time, the various Omicron subvariants ’ have all been similar to one another.
In general, Crotty says, there’s a limit to how low incubation periods can go. The SARS-CoV-2 virus works by invading human cells and using them to make numerous copies of itself. SARS-CoV-2 has a long genome that takes a while to copy, so Crotty doubts its incubation period will get much shorter than it already has. Viruses like measles and varicella (which causes chickenpox) on average take longer than a week to incubate, so, by comparison, a three-day incubation period is already pretty fast.
More Must-Reads from TIME
- How Joe Biden Leads
- Do Less. It’s Good for You
- There's Something Different About Will Smith
- What Animal Studies Are Revealing About Their Minds—and Ours
- What a Hospice Nurse Wants You to Know About Death
- 15 LGBTQ+ Books to Read for Pride
- TIME100 Most Influential Companies 2024
- Want Weekly Recs on What to Watch, Read, and More? Sign Up for Worth Your Time
Write to Jamie Ducharme at [email protected]
Language selection
- Français fr
COVID-19: Symptoms, treatment, what to do if you feel sick
- Current situation
- Symptoms and treatment
- Prevention and risks
- Canada's response
- Guidance documents
Join the effort to limit the spread of COVID-19
Get a COVID-19 test near you
On this page
Covid-19 symptoms, if you have severe symptoms, what to do if you’re sick or were exposed, caring for others, treating covid-19, long-term symptoms.
Symptoms of COVID-19 can vary:
- from person to person
- in different age groups
- depending on the COVID-19 variant
Some of the more commonly reported symptoms include:
- sore throat
- new or worsening cough
- shortness of breath or difficulty breathing
- temperature equal to or more than 38°C
- feeling feverish
- fatigue or weakness
- muscle or body aches
- new loss of smell or taste
- abdominal pain, diarrhea and vomiting
- feeling very unwell
If you don’t feel well or if you have any symptoms, even if mild, assume you may have COVID-19. Immediately isolate at home and away from others. Check with your local public health authority for more advice, including where and how to get tested if recommended.
You may be infected but not have symptoms. However, you can still spread the virus to others. You may:
- develop symptoms later (be pre-symptomatic)
- never develop symptoms (be asymptomatic)
If you’ve been in contact with someone who has COVID-19, contact your local public health authority for advice on what to do next.
Learn more about:
- Testing for COVID-19: When to get tested and testing results
- COVID-19: Contact your local public health authority
Start of symptoms
You may start experiencing symptoms anywhere from 1 to 14 days after exposure. Typically, symptoms appear between 3 and 7 days after exposure.
Vaccination prevents severe illness
Vaccination is one of the most effective ways to protect our families, communities and ourselves against COVID-19. Evidence indicates that the vaccines used in Canada are very effective at preventing severe illness, hospitalization and death from COVID-19.
However, vaccines are not 100% effective and you may still become infected with or without symptoms.
- Vaccines for COVID-19: How to get vaccinated
Public health measures
When layered together, public health measures are effective in reducing the spread of COVID-19, including variants of concern.
Regardless of your vaccination status, you should continue to:
- follow the advice of your local public health authority
- layer multiple individual public health measures to protect yourself and others
- COVID-19: Provincial and territorial resources
- COVID-19: Individual public health measures
Call 911 or your local emergency number if you develop severe symptoms , such as:
- trouble breathing or severe shortness of breath
- persistent pressure or pain in the chest
- new onset of confusion
- difficulty waking up or staying awake
- pale, grey or blue-coloured skin, lips or nail beds
Follow instructions for safe transport if taking an ambulance or a private vehicle to a hospital or clinic.
It’s important that you continue to follow the recommendations and restrictions of your local public health authority on quarantine or isolation if you:
- may have COVID-19 (for example, you feel sick or have been exposed)
- have tested positive for COVID-19
If you have to quarantine or isolate, follow appropriate precautions to reduce the risk of illness spreading within your home. If you don’t have somewhere safe to isolate, contact your local public health authority for available options.
Adults and children with mild COVID-19 symptoms can stay at home while recovering. You don’t need to go to the hospital if symptoms are mild.
Check with your local public health authority about quarantine or isolation periods, and reporting.
Choose your local public health authority:
- British Columbia
- New Brunswick
- Newfoundland and Labrador
- Northwest Territories
- Nova Scotia
- Prince Edward Island
- Saskatchewan
- Safe Voluntary Isolation Sites Program
You may be caring for someone at home who has or may have COVID-19. If so, you should follow the appropriate precautions to reduce the risk of illness spreading within your home.
- COVID-19: What to do if you or someone in your home is sick
If you’re concerned about your symptoms, consult your health care provider. They may recommend steps or medications you can take to relieve some of your symptoms, like fever and cough.
Follow the advice of your health care provider, who may prescribe treatments.
- COVID-19 treatments
Some people who become infected with COVID-19 may experience long-term symptoms, even after they recover from their initial infection. This is sometimes called post COVID-19 condition or long COVID. It has also been called post-acute COVID-19 syndrome (PACS) or long haul COVID.
Studies are underway to further understand what causes post COVID-19 condition and how to diagnose and treat it.
If you think you have this condition, talk to your health care provider about how to manage your symptoms.
- Post COVID-19 condition (long COVID)
Related links
- Digital factsheets, printable posters and shareable videos on COVID-19 (multilingual products available)
- COVID-19: Social media and promotional resources for Health Canada and Public Health Agency of Canada
Page details
- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Supplements
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
- Considerations Ahead of Testing
- At-Home Test Accuracy
- PCR vs. Rapid Tests
- Testing Options for Breakthrough Cases
- Positive Result: What to Do Next
- Does Insurance Cover At-Home COVID Tests?
- Best At-Home Tests
- Retesting After Having COVID

Do You Need to Retest After a Positive COVID-19 Result?
- Positive for COVID-19?
- Do I Need to Retest?
- Positive PCR Test?
- Positive Antibody Test?
- Retest After Re-Exposure?
- Mandatory COVID Testing?
- Signs of Long COVID
- Next in At-Home COVID Test Guide Understanding At-Home COVID Test Accuracy
If you test positive for COVID-19 , you don't need to retest if your symptoms have cleared or are improving. However, you should take steps to prevent the spread of COVID-19 when you're sick. This may include staying away from others if you have a fever and/or taking additional precautions, such as wearing a well-fitting face mask and washing your hands often.
This article explains when to retest after a positive COVID test and explores special situations where retesting may be valuable.
AzmanL / Getty Images
What to Do If You Test Positive for COVID-19
If you test positive for COVID-19, the Centers for Disease Control and Prevention (CDC) recommends that you:
- Take steps to prevent the spread of COVID-19.
- Monitor your symptoms.
- Talk to a healthcare provider about treatment if you are at risk for severe illness.
According to the CDC, to reduce the chances of spreading COVID-19, you should:
- Isolate at home until you've been fever-free for 24 hours (without taking fever-reducing medication) AND your symptoms are mild and improving.
- Take additional precautions for five days following isolation, such as wearing a well-fitting mask, keeping a distance from others, and washing your hands often.
Is It Possible to be Infected With Two Different COVID-19 Variants at the Same Time?
It's not common, but there have been case reports of people who have been diagnosed with two COVID variants at the same time.
Do I Need to Retest After Getting COVID-19?
If you follow the CDC's guidance on preventing respiratory viruses when you're sick, there is generally no need to retest yourself for COVID-19.
What If a PCR Test Is Positive?
There are different tests used to detect COVID-19.
Antigen tests, available over the counter, detect proteins on the surface of the virus itself. Another test called polymerase chain reaction (PCR) , performed in a lab, detects the genetic material of the virus and is often used to confirm a positive antigen test result.
Antigen tests are far less sensitive than PCRs. With a PCR, you can continue to test positive for weeks or even months after an antigen test delivers a negative result.
So if you are re-testing to see if your infection has passed, a positive PCR does not mean that you are contagious. Although minute amounts of the virus may be detected, they may not be at levels capable of infecting others.
What If an Antibody Test Is Positive?
In addition to antigen and PCR tests, there are antibody tests that detect proteins produced by the immune system in response to COVID-19.
Despite being given Emergency Use Authorization (EUA) by the Food and Drug Administration (FDA) in the early part of the pandemic, COVID antibody tests are not used in the same way today.
According to the CDC:
- Antibody tests should not be used to determine if someone has COVID-19.
- Antibody tests should not be used to assess a person's immunity to COVID-19 given that scientists do not know how long or robust these protective antibodies are.
COVID antibody tests have other limitations. Among them, it can take one to three weeks before there are enough antibodies for the test to detect. By that time, many other people could have been infected.
Today, antibody tests are used for population-based research. They are also sometimes used to aid in the diagnosis of multisystem inflammatory syndrome (MIS) , a rare but severe complication of COVID-19 in adults and children.
Should I Retest If Re-Exposed to COVID?
If you tested positive for COVID-19 within 90 days and have been re-exposed to the virus, you may or may not need to be retested. It depends on how long ago you tested positive and whether or not you have symptoms.
According to the CDC, you should be retested if:
- You tested positive for COVID within 30 days and have COVID symptoms.
- You tested positive for COVID within 31 to 90 days and have COVID symptoms.
- You tested positive for COVID within 31 to 90 days and do not have COVID symptoms.
Antigen testing is recommended. If the result is negative, repeat testing should be done.
You should NOT be retested if you tested positive for COVID within 30 days and do not have COVID symptoms.
Window Period for Antigen Testing
The window period for a COVID antigen test is five days. This means that it takes that amount of time for the virus level to be high enough that a test can detect it. Testing before then may lead to a false-negative result .
Can My Employer Require COVID Retesting?
Several workplaces have implemented COVID-19 screening to prevent the spread of the virus. However, the CDC advises against policies that require employees to have a negative COVID result before they can return to work.
Instead, the CDC recommends the same isolation and masking policies it has in place for the general population.
According to the Americans with Disabilities Act (ADA), employers who put mandatory COVID-19 testing in place must ensure that the testing is job-related and consistent with a business necessity. The purpose should be to identify current infections.
Do I Need to Retest a Positive COVID Test for Travel?
You'll need to check the testing requirements for your destination. You may need to show a negative COVID-19 test to enter a country or come back to the country you traveled from. If you test positive for COVID-19 while you are traveling, you will need to follow the guidelines for isolation, testing, and treatment where you are.
Does a Continued Positive Result Mean I Have Long COVID?
Long COVID , also known as post-COVID syndrome, is a chronic condition in which people continue to have symptoms three months after the onset of the initial symptoms or a positive COVID test result .
Symptoms of long COVID may include:
- Shortness of breath
- Joint or muscle pain
- Difficulty concentrating
- Sleep problems
- Mood changes
- Changes in smell or taste
- Changes in the menstrual cycle
Long COVID is diagnosed based on clinical signs and symptoms. There are no tests used to diagnose the syndrome, and repeat testing has no value in determining whether or not you have long COVID.
The CDC does not recommend repeat COVID-19 testing for those who have followed guidance on preventing the spread of the virus and whose symptoms are improving or cleared.
The CDC also does not recommend repeat testing for returning to work. Instead, workplaces should adhere to the same isolation/masking recommendations for the general population.
The information in this article is current as of the date listed, which means newer information may be available when you read this. For the most recent updates on COVID-19, visit our coronavirus news page .
Centers for Disease Control and Prevention. COVID-19 testing: What you need to know .
Centers for Disease Control and Prevention. Preventing spread of respiratory viruses when you’re sick .
Samoilov, Kaptelova, Bukharina, Shipulina, Korneenko, Saenko, Lukyanov, Grishaeva, Ploskireva, Speranskaya, & Akimkin. (2021). Case report: change of dominant strain during dual SARS-CoV-2 infection . BMC Infectious Diseases , 21 (1), 1–8. doi:10.1186/s12879-021-06664-w
University of Chicago Medicine. COVID-19 testing: When to test, how accurate are home tests and more .
Centers for Disease Control and Prevention. Interim guidance for SARS-CoV-2 testing in non-healthcare workplaces .
U.S. Equal Employment Opportunity Commission. What you should know about COVID-19 and the ADA, the Rehabilitation Act, and other EEO laws .
Centers for Disease Control and Prevention. Travel .
World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021 .
Centers for Disease Control and Prevention. Post-COVID conditions .
Yomogida K, Zhu S, Rubino F, Figueroa W, Balanji N, Holman E. Post-acute sequelae of SARS-CoV-2 infection among adults aged ≥18 years - Long Beach, California, April 1-December 10, 2020 . MMWR Morb Mortal Wkly Rep . 2021;70(37):1274-1277. doi:10.15585/mmwr.mm7037a2
By Christine Zink, MD Dr. Zink is a board-certified emergency medicine physician with expertise in the wilderness and global medicine.
Official websites use .gov
A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
CDC updates and simplifies respiratory virus recommendations
Recommendations are easier to follow and help protect those most at risk
For Immediate Release: Friday, March 1, 2024 Contact: Media Relations (404) 639-3286
CDC released today updated recommendations for how people can protect themselves and their communities from respiratory viruses, including COVID-19. The new guidance brings a unified approach to addressing risks from a range of common respiratory viral illnesses, such as COVID-19, flu, and RSV, which can cause significant health impacts and strain on hospitals and health care workers. CDC is making updates to the recommendations now because the U.S. is seeing far fewer hospitalizations and deaths associated with COVID-19 and because we have more tools than ever to combat flu, COVID, and RSV.
“Today’s announcement reflects the progress we have made in protecting against severe illness from COVID-19,” said CDC Director Dr. Mandy Cohen. “However, we still must use the commonsense solutions we know work to protect ourselves and others from serious illness from respiratory viruses—this includes vaccination, treatment, and staying home when we get sick.”
As part of the guidance, CDC provides active recommendations on core prevention steps and strategies:
- Staying up to date with vaccination to protect people against serious illness, hospitalization, and death. This includes flu, COVID-19, and RSV if eligible.
- Practicing good hygiene by covering coughs and sneezes, washing or sanitizing hands often, and cleaning frequently touched surfaces.
- Taking steps for cleaner air , such as bringing in more fresh outside air, purifying indoor air, or gathering outdoors.
When people get sick with a respiratory virus, the updated guidance recommends that they stay home and away from others. For people with COVID-19 and influenza, treatment is available and can lessen symptoms and lower the risk of severe illness. The recommendations suggest returning to normal activities when, for at least 24 hours, symptoms are improving overall, and if a fever was present, it has been gone without use of a fever-reducing medication.
Once people resume normal activities, they are encouraged to take additional prevention strategies for the next 5 days to curb disease spread, such as taking more steps for cleaner air, enhancing hygiene practices, wearing a well-fitting mask, keeping a distance from others, and/or getting tested for respiratory viruses. Enhanced precautions are especially important to protect those most at risk for severe illness, including those over 65 and people with weakened immune systems. CDC’s updated guidance reflects how the circumstances around COVID-19 in particular have changed. While it remains a threat, today it is far less likely to cause severe illness because of widespread immunity and improved tools to prevent and treat the disease. Importantly, states and countries that have already adjusted recommended isolation times have not seen increased hospitalizations or deaths related to COVID-19.
While every respiratory virus does not act the same, adopting a unified approach to limiting disease spread makes recommendations easier to follow and thus more likely to be adopted and does not rely on individuals to test for illness, a practice that data indicates is uneven.
“The bottom line is that when people follow these actionable recommendations to avoid getting sick, and to protect themselves and others if they do get sick, it will help limit the spread of respiratory viruses, and that will mean fewer people who experience severe illness,” National Center for Immunization and Respiratory Diseases Director Dr. Demetre Daskalakis said. “That includes taking enhanced precautions that can help protect people who are at higher risk for getting seriously ill.”
The updated guidance also includes specific sections with additional considerations for people who are at higher risk of severe illness from respiratory viruses, including people who are immunocompromised, people with disabilities, people who are or were recently pregnant, young children, and older adults. Respiratory viruses remain a public health threat. CDC will continue to focus efforts on ensuring the public has the information and tools to lower their risk or respiratory illness by protecting themselves, families, and communities.
This updated guidance is intended for community settings. There are no changes to respiratory virus guidance for healthcare settings.
### U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
Whether diseases start at home or abroad, are curable or preventable, chronic or acute, or from human activity or deliberate attack, CDC’s world-leading experts protect lives and livelihoods, national security and the U.S. economy by providing timely, commonsense information, and rapidly identifying and responding to diseases, including outbreaks and illnesses. CDC drives science, public health research, and data innovation in communities across the country by investing in local initiatives to protect everyone’s health.
To receive email updates about this page, enter your email address:
- Data & Statistics
- Freedom of Information Act Office
Exit Notification / Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private website.
- My View My View
- Following Following
- Saved Saved
Pfizer's Paxlovid fails as 15-day treatment for long COVID, study finds
- Medium Text
- Company Pfizer Inc Follow
Sign up here.
Reporting by Michael Erman Editing by Bill Berkrot
Our Standards: The Thomson Reuters Trust Principles. New Tab , opens new tab

Business Chevron

Australia jobs climb 39,700 in May, unemployment dips to 4%
Australian employment outpaced expectations in May as firms took on more full-time workers, while the jobless rate dipped in a sign the labour market remains resilient to high interest rates and weak consumer demand.

Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
Register to vote Register by 18 June to vote in the General Election on 4 July.
COVID-19 Response: Living with COVID-19
- Cabinet Office
Updated 6 May 2022
Applies to England

© Crown copyright 2022
This publication is licensed under the terms of the Open Government Licence v3.0 except where otherwise stated. To view this licence, visit nationalarchives.gov.uk/doc/open-government-licence/version/3 or write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or email: [email protected] .
Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned.
This publication is available at https://www.gov.uk/government/publications/covid-19-response-living-with-covid-19/covid-19-response-living-with-covid-19
1. Introduction
The Government’s aim throughout the COVID-19 pandemic has been to protect the lives and livelihoods of citizens across the United Kingdom (UK). This document sets out how the Government has and will continue to protect and support citizens by: enabling society and the economy to open up more quickly than many comparable countries; using vaccines; and supporting the National Health Service ( NHS ) and social care sector. It also sets out how England will move into a new phase of managing COVID-19. The Devolved Administrations will each set out how they will manage this transition in Scotland, Wales and Northern Ireland.
The global pandemic is not yet over and the Government’s Scientific Advisory Group for Emergencies ( SAGE ) is clear there is considerable uncertainty about the path that the pandemic will now take in the UK. [footnote 1] This document therefore also sets out how the Government will ensure resilience, maintaining contingency capabilities to deal with a range of possible scenarios.
COVID-19 response: Roadmap to the present day
Vaccines have enabled the gradual and safe removal of restrictions on everyday life over the past year, and will remain at the heart of the Government’s approach to living with the virus in the future. The Government and the NHS , with the help of volunteers, has delivered one of the largest vaccination programmes in history.
Figure 1: Vaccines: UK Cumulative vaccinations [footnote 2]
Area chart of the percentage of the UK population aged 12+ who have received first doses, second doses and booster doses of a COVID-19 vaccine. On the 14 of September 2021, 84% of the population had had a first dose, 77% had had a second dose, and the booster programme had not yet started. On 16 February 2022, this had increased to 91% of the over 12 population who had received a first dose, 85% who had received a second dose and 66% who had received a booster dose.
The speed of the vaccine rollout put the UK in a strong position. The UK was the first country in the world to authorise and deploy the Pfizer and Oxford / AstraZeneca COVID-19 vaccines. [footnote 3] The UK was the first major European economy and first G20 member to vaccinate 50% of its population with at least one dose, [footnote 4] and to provide boosters to 50% of the population. [footnote 5] Moreover, on JCVI advice, the UK prioritised those at highest risk from COVID-19 for vaccination early in the roll-out. Although other countries now exceed the UK’s proportion of the total population vaccinated, the speed and highly targeted nature of the vaccination programme had a direct impact on the Government’s ability to open up the economy and ease social restrictions sooner than other comparator countries, without placing the NHS under unsustainable pressure.
As a result of the vaccine rollout, the Government was able to ease restrictions in England through the first half of 2021 - following the plan set out in the Roadmap in the Government’s ‘COVID-19 Response: Spring 2021’ publication. [footnote 6] The nationwide lockdown introduced in January 2021 was lifted in four steps, with decisions to progress based on data not dates. Each step was taken at least five weeks apart, allowing time to assess the impact of the previous step against four key tests before taking the next step.
On 19 July 2021, the Government removed most restrictions in England at step 4 of the Roadmap and, in doing so, opened up earlier than many other comparable countries. The Government made a deliberate choice to do so at this point as it coincided with the end of the school term and meant that restrictions were removed over the summer period when more activities take place outdoors and there is less pressure on the NHS .
In September 2021 the Government published its ‘COVID-19 Response: Autumn and Winter Plan’, setting out a comprehensive plan for managing the virus over the colder months. [footnote 7] Plan A for England relied on booster vaccinations, testing and isolation, guidance on safer behaviours and measures at the border. The publication also outlined a Plan B which could be deployed later in the winter if the situation deteriorated. The measures in Plan B – mandatory face coverings, working from home guidance and COVID-19 certification – were designed to reduce transmission while minimising economic and social impacts.
From September to November 2021, the Government:
- a. Extended the vaccine programme to children aged between 12 and 15 and started the booster campaign for those 50 and over and in high risk groups;
- b. Maintained a lower level of restrictions than most European comparator countries; and
- c. Managed relatively high levels of Delta infections without placing the NHS at risk of unsustainable pressures.
On 24 November, scientists in South Africa reported a new variant with troubling yet uncertain characteristics to the World Health Organization ( WHO ). This was subsequently named the Omicron variant. [footnote 8] The UK was one of the first countries to respond, initially through travel restrictions, then through accelerating and extending the COVID-19 vaccine booster campaign. The Government was in a position to implement Plan B measures in England at short notice as a result of the plans developed for managing the virus over the autumn and winter period.
Although the Omicron variant drove prevalence of the virus to an unprecedented high, adherence to Plan B, wider behaviour change and large-scale testing appeared to slow the growth sufficiently to buy time for the extended booster campaign. This trend was improved by high and sustained vaccine-induced protection in the population against severe disease, and a decrease in severity found in the Omicron variant, which meant that hospitalisation rates remained lower than in previous waves. In particular, the proportion of patients being admitted to intensive care and requiring mechanical ventilation remained lower, with rates declining even when prevalence had increased. [footnote 9] This was in part also due to better clinical understanding of the disease.
During this period, the public continued to show willingness to get vaccinated and boosted, to test and self-isolate if they had symptoms or tested positive, and to follow behaviours and actions that limit methods of transmission.
The people of the UK also owe much to the NHS and its brilliant staff - as well as to providers and staff in adult social care - who throughout the pandemic have drawn deeply on their professionalism, skills and training to do their very best for patients and care recipients. This includes hugely ramping up the booster campaign last winter in response to the Omicron variant. This response played a key role in avoiding the kind of stringent restrictions seen in other countries this winter. Against this backdrop, the Government reverted to Plan A on 27 January, maintaining England as one of the most open countries in Europe.
COVID-19: Future outlook
There are a range of possible futures for the course of the pandemic. SAGE has recently considered four scenarios describing plausible outcomes, though these are not predictions. [footnote 10] All scenarios assume that a more stable position will eventually be reached over several years. In the ‘reasonable best case’ there may be a comparatively small resurgence in infections during autumn/winter 2022-23, and in the ‘reasonable worst case’ a very large wave of infections with increased levels of severe disease. The ‘optimistic central’ and ‘pessimistic central’ scenarios are considered the most likely.
The emergence of new variants will be a significant factor in determining the future path of the virus. New variants of COVID-19 will continue to emerge. [footnote 11] This could include variants that render vaccines less effective, are resistant to antivirals, or cause more severe disease. [footnote 12] The pathway to greater stability will also be affected by the use of vaccination and available treatments.
The term ‘endemic’ is sometimes used to denote when a more steady or more predictable state has been reached but it does not mean that a virus will necessarily circulate at low levels or that outbreaks cannot or will not occur. Given the uncertainty, the Government will need to continue to monitor how COVID-19 is behaving and be ready to respond to resurgences and new variants.
Once COVID-19 becomes endemic it should be possible to respond to the virus in a similar way to other existing respiratory illnesses, through sustainable public health measures. The transition to an endemic state will be highly dynamic and affected by the international situation. It will occur at different times globally due to differences in the spread of the disease and access to vaccines.
The Government expects that the population’s defences against new variants will continue to strengthen as immunity increases through advances in vaccine technology and repeated exposure to the virus. As with other human coronaviruses, children will very likely be exposed to COVID-19 during their childhood and future generations are likely to become progressively more protected by the combination of vaccination and infection.
Studying other infectious diseases can offer insights into the future of COVID-19, though comparisons are imperfect. While a different disease to COVID-19, the most common comparison is to influenza. Both viruses can result in severe illness and complications and are thought to spread in similar ways. The virus that causes COVID-19 is far more contagious and can cause more serious illness, even in otherwise healthy people. Influenza is managed through ongoing surveillance, annual vaccination and annual public messaging, including campaigns to increase vaccine uptake and the ‘Catch it, Bin it, Kill it’ campaign to reduce transmission from coughs and sneezes. Influenza still produces regular winter epidemics, causing pressure on the NHS every winter. The interaction of future COVID-19 waves with other respiratory infections, like influenza, will be important to monitor. Co- or sequential circulation could lead to an increased or longer period of pressure on healthcare services.
Over time, though hard to predict, it is likely that COVID-19 will become a predominantly winter seasonal illness with some years seeing larger levels of infection than others. This may take several years to occur and waves of infection may occur during winter or at other times in the year.
COVID-19: Future response
The Government’s objective in the next phase of the COVID-19 response is to enable the country to manage COVID-19 like other respiratory illnesses, while minimising mortality and retaining the ability to respond if a new variant emerges with more dangerous properties than the Omicron variant, or during periods of waning immunity, that could again threaten to place the NHS under unsustainable pressure.
To meet this objective, the Government will structure its ongoing response around four principles:
- a. Living with COVID-19: removing domestic restrictions while encouraging safer behaviours through public health advice, in common with longstanding ways of managing most other respiratory illnesses;
- b. Protecting people most vulnerable to COVID-19: vaccination guided by Joint Committee on Vaccination and Immunisation ( JCVI ) advice, and deploying targeted testing;
- c. Maintaining resilience: ongoing surveillance, contingency planning and the ability to reintroduce key capabilities such as mass vaccination and testing in an emergency; and
- d. Securing innovations and opportunities from the COVID-19 response, including investment in life sciences.
Vaccines underpin all of these principles and form the basis of the Government’s strategy for living with COVID-19. Effective vaccines have allowed the economy and society to reopen and the country’s ability to live with the virus in the future will continue to depend on deeper and broader population immunity conferred by vaccines and infections. In line with this:
- a. The Government will continue to be guided by JCVI advice on deploying vaccinations. This includes the recent decision to offer vaccination to all 5-11 year olds later in the spring. Subject to JCVI advice, further vaccinations (boosters) may be recommended for people who are most vulnerable to COVID-19 this autumn and, ahead of that, a spring booster for groups JCVI consider to be at particularly high risk.
- b. To enable any further vaccination programme, if necessary, the Government has procured enough doses of vaccine to anticipate a wide range of possible JCVI recommendations. The UK’s procurement approach will continue to consider a range of long term contingency plans to ensure adequate protection is always available for those who need it and to respond quickly in an emergency.
- c. The Government has secured contracts with vaccine manufacturers that secure UK access to the most up-to-date vaccines - including protection against emerging variants. The UK remains an attractive destination for life sciences investment, and the Government is committed to supporting UK resilience for future pandemics, by considering how to support research, development and manufacturing capability.
- d. The Government will help build global resilience to COVID-19 by meeting its commitment to donate 100 million vaccine doses by June 2022 and by continuing to support the ACT Accelerator. The Government is also working domestically and with the G7, G20, and international partners to reduce the impact of future pandemics through the 100 Days Mission. [footnote 13]
Work is underway across the health and care system to consider how vaccines will be procured, prioritised and deployed in the future. The Government’s aim is to capture the best learning from the pandemic response.
2. COVID-19: Data and impacts
Vaccination, infection and hospitalisation rates.
Booster doses of a COVID-19 vaccine provide good protection against severe disease and hospitalisation for the Omicron variant. Following two doses of the Pfizer or AstraZeneca vaccines, a Pfizer booster initially gives around 90% protection against hospitalisation, though this effect wanes over time. [footnote 14] Similarly, a Moderna booster gives 90 to 95% protection against hospitalisation up to 9 weeks after vaccination. [footnote 15]
Vaccine uptake
In England, over 65% of all those aged 12 and over have received a booster, increasing to 66% across the UK. [footnote 16] [footnote 17] Vaccination rates are even higher among those most vulnerable to COVID-19 - who were prioritised for vaccination - and in England, over 93% of those aged 70 and over have received a booster. [footnote 18]
Figure 2: UK population COVID-19 vaccine coverage, by dose, of those aged 12 and over as of 16 February 2022 [footnote 19]
Pie chart showing the percentage share of the population over 12 years old who are unvaccinated or received one, only two or three doses of a COVID-19 vaccination. As of 16 February 2022, 9% of the over 12 population were unvaccinated, 6% had received only one dose, 19% had received only two doses and 66% had received a booster dose.
Since the start of September 2021 over 1.5 million adults over the age of 18 in England have come forward for a first dose of COVID-19 vaccine, long after receiving an initial offer. [footnote 20] As a result, the percentage of the population aged over 18 in England who have received at least one dose has increased from 88 to 92%. [footnote 21] However, over 3.4 million people in England aged 18 and older remain unvaccinated. [footnote 22]
Whilst vaccine uptake has increased across many groups, it remains considerably lower amongst certain communities. The UK Health Security Agency ( UKHSA ) data show booster uptake was lowest amongst Black and Pakistani adults (below 35%). [footnote 23] Data also shows that adults living in the most deprived areas of England also had lower booster uptake (53%) than those living in the least deprived areas (84%). [footnote 24] Analysis also shows that disparities in vaccine uptake are also present in younger age groups: only 39% of 18 to 24 year olds in England have received a booster dose, much lower than for older age groups. [footnote 25]
The proportion of 12 to 15 year olds who have received at least one dose of vaccine is lowest in Gypsy/Roma, Traveller Irish, Black Caribbean and Black African groups (all below 30%), with a 63 percentage point difference between the most and least vaccinated ethnic groups. [footnote 26] There is also large variation in vaccine coverage by deprivation in 12 to 15 year olds. In the least deprived areas in England 70% of this age group have received at least one dose, compared to 36% in the most deprived. [footnote 27]
Overall, the accumulation of immunity, as well as the use of effective treatments, means the link between COVID-19 infections and progression to severe disease is substantially weaker than in earlier phases of the pandemic. Patients in hospital per 100 infections have remained low over the last six months, with less than 1 hospitalisation per 100 infections compared to above 4 per 100 infections during the Alpha variant peak. [footnote 28] [footnote 29] Lower hospitalisation is partly due to improved treatments but also in part attributable to the lowered virulence of the Omicron variant.
Figure 3: UK: Patients in hospital with COVID-19 per 100 infections using ONS COVID-19 Infection Survey estimates [footnote 30] [footnote 31]
Line chart of the ratio of patients in hospital per infection. In early 2021 there were nearly 5 patients for every 100 infections, this has dropped to less than 0.5.
The ratio is lagged by 8 days (the difference in the peak infections to peak admissions), reflecting the estimated number of infections that occurred 8 days ago that went on to be admitted to hospital on a given date.
Reporting on COVID-19
As testing reduces and the Government’s approach to managing COVID-19 further evolves, UKHSA will keep the content and frequency of reporting on COVID-19 under close review - including the Gov.uk Dashboard - to ensure that statistics are being produced with the appropriate level of quality and transparency, and remain useful and relevant as per the Code of Practice for Statistics.
Impact of COVID-19 response to date on the economy and society
Since March 2020, to reduce transmission, protect the NHS from unsustainable pressure and to reduce mortality, the Government has had to introduce stringent measures by restricting social and economic activity.
The measures introduced were necessary because COVID-19 was a new disease to which the population had no immunity, and for which there was no readily available treatment. However, the measures introduced had extraordinarily high social and economic costs with unprecedented impacts on individuals and families, public services and private businesses.
In particular, the health and education sectors have been significantly affected, as well as the provision of other public services such as the court system. The pandemic has also caused a period of unparalleled global economic uncertainty. Restrictions to control the virus - including social distancing, business closures and reduced international travel - on top of voluntary behaviour change, had significant economic costs, and disrupted the delivery of critical private and public sector services.
Impacts on health, education and public services
During the pandemic, over 720,000 patients have been admitted to hospital with COVID-19, [footnote 32] and over 160,000 people have now died within 28 days of a positive test in the UK. [footnote 33] Caring for this number of patients has restricted the ability of the NHS to provide other types of care. As a result the NHS elective backlog has reached a record high and waiting times for ambulances and emergency care have substantially increased.
The provision of other public services has also been significantly affected. The court backlog increased substantially during the pandemic [footnote 34] and restricting face-to-face education has had significant adverse impacts on children and young people’s learning, development and mental health. Pupils and students from disadvantaged backgrounds experienced greater losses in learning than their more affluent peers as a result of the pandemic. [footnote 35] There is clear evidence that time out of education can be detrimental to children and young people’s future prospects and earning potential, with implications also for long-term productivity.
Mental health and well-being have also been negatively impacted. Self-reported measures of personal well-being dropped to record lows during the first and second waves, with some groups experiencing particularly poor or deteriorating mental health - including women, young people, disabled people, those in deprived neighbourhoods, certain ethnic minority groups and those who experienced local lockdowns. [footnote 36] There was a marked increase in the number of under 18s referred to specialist care for issues such as self-harm and eating disorders in 2021. [footnote 37] Reports of domestic abuse increased during lockdown periods. [footnote 38]
Impacts on the economy
The pandemic and associated non-pharmaceutical interventions ( NPIs ) created significant economic disruption and drove the largest recession on record, with the UK economy contracting by 9.4% in 2020. [footnote 39]
As experience allowed for improved understanding of the impact of restrictions, businesses, consumers and the Government adapted. For example, the Government was able to deploy more targeted interventions. Compared to pre-pandemic levels (February 2020), output was 25% lower during the first lockdown (April 2020), and 7% lower in November 2020, coinciding with much of the second lockdown and 8% lower at the height of the third lockdown (January 2021). [footnote 40]
The Government took unprecedented steps to support the economy through the pandemic. The Government has provided around £400 billion of direct support for the economy through the pandemic to date. [footnote 41] This has helped to safeguard jobs and businesses in every region and nation of the UK, and underpinned the faster than expected economic bounce back that occurred when restrictions were lifted. The Coronavirus Job Retention Scheme succeeded in supporting 11.7 million jobs and 1.3 million employers across the UK and the Self-Employment Income Support Scheme supported nearly 3 million self-employed individuals. [footnote 42]
As restrictions were lifted in 2021, supported by the vaccine rollout, consumer activity increased, driving recovery across the economy. As uncertainty declined, business confidence and investment also began to recover. 2021 saw faster than anticipated growth, with the economy regaining its pre-pandemic size in November 2021. [footnote 43] The emergence of the Omicron variant, workforce absences from illness and isolation, and Plan B measures in England impacted economic activity in recent months, with GDP falling 0.2% in December 2021. [footnote 44]
Workforce absences due to illness and self-isolation have weighed on economic growth in periods of particularly high prevalence during the Delta and Omicron waves. Workforce absences disproportionately impacted those less able to work from home, who were more likely to be young, on lower incomes, or from certain ethnic minority groups. [footnote 45] Changes to self-isolation policy helped to mitigate these impacts while accepting a higher risk of transmission.
Government action has supported a strong recovery in the labour market. The number of payrolled employees in January 2022 was 436,000 above February 2020 levels. [footnote 46] Vacancies remained at a record level in the 3 months to January 2022, standing at 1.3 million. [footnote 47]
Following the easing of restrictions in summer 2021, supply pressures due to COVID-19 have acted as a constraint on output in many countries including the UK. This has been a result of: restrictions on people’s ability to work; factory closures globally; and elevated consumer demand for goods. While supply pressures remain acute, there are some initial signs of easing with shipping costs falling from October 2021. However, the possibility of further outbreaks internationally and different approaches to COVID-19 taken by different countries could present further risks to the UK economy.
3. Living with COVID-19
The past 2 years have seen many necessary restrictions imposed on everyday life to manage COVID-19, but these have come with a huge toll on wellbeing and economic output. Scientists (including virologists, epidemiologists, clinicians, and many others) and the Government now understand more about COVID-19, how it behaves and how it can be treated. As the virus continues to evolve, it will be important to continue to add to this understanding.
Living with and managing the virus will mean maintaining the population’s wall of protection and communicating safer behaviours that the public can follow to manage risk. The Government will move away from deploying regulations and requirements in England and replace specific interventions for COVID-19 with public health measures and guidance.
The Government is able to take this step now because of the success of the vaccination programme, and the suite of pharmaceutical tools the NHS can deploy to treat people who are most vulnerable to COVID-19 and the most severely ill (see chapter 4). The Government can only take these steps because it will retain contingency capabilities and will respond as necessary to further resurgences or worse variants of the virus (see chapter 5).
Removing the last domestic restrictions
The Government will remove remaining domestic restrictions in England, subject to appropriate parliamentary scrutiny.
From 24 February, the Government will:
- a. Remove the legal requirement to self-isolate following a positive test. Adults and children who test positive will continue to be advised to stay at home and avoid contact with other people. After 5 days, they may choose to take a Lateral Flow Device ( LFD ) followed by another the next day - if both are negative, and they do not have a temperature, they can safely return to their normal routine. Those who test positive should avoid contact with anyone in an at risk group, including if they live in the same household. There will be specific guidance for staff in particularly vulnerable services, such as adult social care, healthcare, and prisons and places of detention.
- b. No longer ask fully vaccinated close contacts and those under the age of 18 to test daily for 7 days, and remove the legal requirement for close contacts who are not fully vaccinated to self-isolate. Guidance will set out the precautions that those who live in the same household as someone who has COVID-19, or who have stayed overnight in the same household, are advised to take to reduce risk to other people. Other contacts of people with COVID-19 will be advised to take extra care in following general guidance for the public on safer behaviours.
- c. End self-isolation support payments and national funding for practical support. The medicine delivery service will no longer be available. People who were instructed to self-isolate before this date will still be able to claim support payments within the next 42 days.
- d. Revoke The Health Protection (Coronavirus, Restrictions) (England) (No. 3) Regulations. Local authorities will continue to manage local outbreaks of COVID-19 in high risk settings as they do with other infectious diseases.
From 24 March, the COVID-19 provisions within Statutory Sick Pay and Employment and Support Allowance regulations will end. People with COVID-19 may still be eligible, subject to the normal conditions of entitlement.
From 1 April, the Government will update guidance setting out the ongoing steps that people with COVID-19 should take to minimise contact with other people. This will align with the changes to testing set out later in this chapter.
Testing, tracing and certification
Testing and tracing have been important throughout the response to COVID-19. The Government’s provision of LFDs enabled people to take a test before meeting family, friends and colleagues, allowing them to protect themselves and others, and breaking chains of transmission. This was particularly important during the period of exceptionally high prevalence driven by the Omicron variant towards the end of 2021. Access to LFDs also enabled contacts of positive cases to test daily in lieu of isolation, reducing the workforce impacts of isolation while identifying positive cases.
However, the Government’s free provision of testing at scale has come at a very significant cost to the taxpayer during the pandemic response. The Testing, Tracing and Isolation ( TTI ) budget in the financial year 2020-21 exceeded that of the Home Office, and the programme cost £15.7 billion in the financial year 2021-22. This level of spending was necessary due to the severe risk posed by COVID-19 when the population did not have a high level of protection.
The population now has much stronger protection against COVID-19 than at any other point in the pandemic, due to the vaccination programme, natural immunity, access to antivirals, and increased scientific and public understanding about how to manage risk. For this reason, the Government now assesses that it is time to transition to focus its COVID-19 response towards guidance, while targeting protection on individuals who are most at risk from the virus. Government spending on COVID-19 will reduce significantly in line with this change.
As immunity levels are high, testing and isolation will play a less important role in preventing serious illness. Some changes to testing have already begun. In January, the recommendation for a confirmatory polymerase chain reaction ( PCR ) test following a positive LFD was changed, and the testing regime in adult social care was also changed to a LFD regime.
The Government will implement further changes to the availability of testing in the coming months.
From 21 February, the Government is removing the guidance for staff and students in most education and childcare settings to undertake twice weekly asymptomatic testing.
From 1 April, the Government will no longer provide free universal symptomatic and asymptomatic testing for the general public in England.
Over 2 billion lateral flow tests have been provided across the UK since 2020. UKHSA continues to have good stock levels and will manage these to provide flexibility in future. Ahead of the end of free universal testing in England, it will be necessary for UKHSA to cap the number of tests distributed each day to manage demand. Given that advice to test has and continues to reduce, the Government urges people only to order what they need.
The Government will help enable COVID-19 tests to be made available for those who wish to purchase them through the private market. Private markets are established in many European countries - including France, Germany, Italy and Spain - and the United States of America. The Government is working with retailers and pharmacies to help establish the private market in testing.
From 1 April, there will be some limited ongoing free testing:
- a. Limited symptomatic testing available for a small number of at-risk groups - the Government will set out further details on which groups will be eligible.
- b. Free symptomatic testing will remain available to social care staff
Contact tracing
From 24 February, routine contact tracing will end. Contacts will no longer be required to self-isolate or advised to take daily tests. Instead, guidance will set out precautions that contacts can take to reduce risk to themselves and other people - and those testing positive for COVID-19 will be encouraged to inform their close contacts so that they can follow that guidance.
Local health teams continue to use contact tracing and provide context-specific advice where they assess this to be necessary as part of their role in managing infectious diseases.
COVID-status certification
From 1 April, the Government will remove the current guidance on domestic voluntary COVID-status certification and will no longer recommend that certain venues use the NHS COVID Pass. The NHS COVID Pass will remain available within the NHS App for a limited period, to support the use of certification in other parts of the UK. The NHS App will continue to allow individuals access to their vaccination status for international travel, as well as their recovery status for travel to those overseas destinations that recognise it.
Safer behaviours
Throughout the pandemic, Government advice and information has been informed by the best scientific evidence available from health agencies, academics, and experts. [footnote 48]
People will continue to be advised that there are safer behaviours they can adopt to reduce the risk of infection. Precautions remain particularly important to those who are at higher risk if they catch COVID-19, although due to advances in vaccination and therapeutics, this group is now better protected. The majority of people previously considered clinically extremely vulnerable are now advised to follow the same general guidance as everyone else as a result of the protection they have received from vaccination.
Individuals can still reduce the risk of catching and passing on COVID-19 by:
- a. Getting vaccinated;
- b. Letting fresh air in if meeting indoors, or meeting outside;
- c. Wearing a face covering in crowded and enclosed spaces, especially where you come into contact with people you do not usually meet, when rates of transmission are high;
- d. Trying to stay at home if you are unwell;
- e. Taking a test if you have COVID-19 symptoms, and staying at home and avoiding contact with other people if you test positive; and
- f. Washing your hands and following advice to ‘Catch it, Bin it, Kill it’.
From 1 April, guidance to the public and to businesses will be consolidated in line with public health advice. There will continue to be specific guidance for those whose immune system means they are at higher risk of serious illness from COVID-19 despite vaccination.
Businesses and other organisations
Employers and businesses have also taken significant steps over the pandemic to mitigate the risks of COVID-19 within their settings. The Government has lifted the majority of legal requirements on businesses, and continues to provide ‘Working Safely’ guidance setting out the steps that employers can take to reduce risk in their workplaces.
From 24 February, workers will not be legally obliged to tell their employers when they are required to self-isolate. Employers and workers should follow Government guidance for those with COVID-19.
From 1 April, the Government will remove the health and safety requirement for every employer to explicitly consider COVID-19 in their risk assessments. The intention is to empower businesses to take responsibility for implementing mitigations that are appropriate for their circumstances. Employers that specifically work with COVID-19, such as laboratories, must continue to undertake a risk assessment that considers COVID-19.
From 1 April, the Government will replace the existing set of ‘Working Safely’ guidance with new public health guidance. Employers should continue to consider the needs of employees at greater risk from COVID-19, including those whose immune system means they are at higher risk of serious illness from COVID-19. The Government will consult with employers and businesses to ensure guidance continues to support them to manage the risk of COVID-19 in workplaces.
Ventilation
The Government will continue to promote and support good ventilation. Employers and businesses should continue identifying poorly ventilated spaces and take steps to improve fresh air flow.
There is increasing evidence of the importance of circulating fresh air in reducing the risk of COVID-19 transmission. Ventilation also helps with reducing transmission of other respiratory infections such as influenza, with some research showing that being in a room with fresh air can in some cases reduce the risk of airborne transmission of COVID-19 by over 70%. [footnote 49] There are also potential wider benefits of good ventilation, for health, concentration, and lower absence rates. [footnote 50] The Government has responded to this evidence through:
- a. Public communications campaigns and comprehensive business guidance on ventilation and fresh air;
- b. Providing over 350,000 CO2 monitors to state-funded education settings backed by £25 million of funding, [footnote 51] and up to 9,000 high efficiency particulate air ( HEPA ) cleaning units for the small number of education settings where poor ventilation could not be quickly rectified; [footnote 52]
- c. Enabling local authorities to use their allocations from the £60 million Adult Social Care Omicron Support Fund, at their discretion, to audit and improve fresh air in adult social care; [footnote 53] and
- d. Completing a ventilation audit of the central government estate.
The Government is also carrying out further ventilation research and the Government’s Chief Scientific Adviser has commissioned a report from the Royal Academy of Engineering on how our built environment could be made more infection resilient, to be published this May. The Government will carefully consider its recommendations, alongside the ongoing research.
Changes at a glance
Today, 21 February the Government is:
- Removing the guidance for staff and students in most education and childcare settings to undertake twice weekly asymptomatic testing.
From 24 February the Government will:
- Remove the legal requirement to self-isolate following a positive test. Adults and children who test positive will continue to be advised to stay at home and avoid contact with other people for at least 5 full days and then continue to follow the guidance until they have received 2 negative test results on consecutive days.
- No longer ask fully vaccinated close contacts and those aged under 18 to test daily for 7 days, and remove the legal requirement for close contacts who are not fully vaccinated to self-isolate.
- End self-isolation support payments, national funding for practical support and the medicine delivery service will no longer be available.
- End routine contact tracing. Contacts will no longer be required to self-isolate or advised to take daily tests.
- End the legal obligation for individuals to tell their employers when they are required to self-isolate.
- Revoke The Health Protection (Coronavirus, Restrictions) (England) (No. 3) Regulations.
From 24 March, the Government will:
- Remove the COVID-19 provisions within the Statutory Sick Pay and Employment and Support Allowance regulations.
From 1 April, the Government will:
- Remove the current guidance on voluntary COVID-status certification in domestic settings and no longer recommend that certain venues use the NHS COVID Pass.
- Update guidance setting out the ongoing steps that people with COVID-19 should take to minimise contact with other people. This will align with the changes to testing.
- No longer provide free universal symptomatic and asymptomatic testing for the general public in England.
- Consolidate guidance to the public and businesses, in line with public health advice.
- Remove the health and safety requirement for every employer to explicitly consider COVID-19 in their risk assessments.
- Replace the existing set of ‘Working Safely’ guidance with new public health guidance.
4. Protecting people most vulnerable to COVID-19
Since March 2020, the medical and scientific community has learned a lot more about COVID-19, what makes someone more or less vulnerable to it, and how to manage the virus in higher risk settings.
At the start of the pandemic very little was known about risk factors from COVID-19 and vaccines were unavailable, so the Government took a precautionary approach and advised ‘clinically extremely vulnerable’ groups to follow shielding advice. These measures were extremely restrictive and often had a significant impact on individuals’ lives and their mental and physical wellbeing, meaning people and their families made considerable sacrifices to stay safe.
Data on COVID-19 related deaths and admissions between December 2020 and June 2021 showed that COVID-19 mortality increased with age (when controlled for vaccination status and other key factors). This same analysis showed that the risk was higher for people with specific clinical conditions such as Down’s syndrome, solid organ transplantation, Dementia, Parkinson’s disease, and neurological conditions. Those living in more deprived areas and from certain ethnic minority groups were also at higher risk of COVID-19 mortality. [footnote 54]
As a result of the success of the Government’s strategy to invest in scientific and medical innovation, the Government has been able to rely more on vaccines and medical treatments, and gradually remove restrictive guidance for those at an increased risk of COVID-19. The shielding programme ended on 15 September 2021.
The Government prioritised those at highest risk from COVID-19 for vaccination by following JCVI advice, and using the COVID-19 Population Risk Assessment. Vaccination has proved to be the most effective way to protect those at increased risk from COVID-19 and everybody should be encouraged to get all doses of the vaccination and boosters for which they are eligible. The Government and UKHSA will continue to communicate to people most vulnerable to COVID-19 about available clinical interventions, including vaccination and treatments, and also testing and public health advice (see previous chapter).
COVID-19 vaccines remain the most important and effective way the public can protect themselves and others from becoming seriously ill or dying from the virus. Vaccines have built a wall of defence around communities across the country, saving countless lives and allowing a phased return to normality. A recent review by UKHSA also showed that people who have had one or more doses of a COVID-19 vaccine are less likely to develop long COVID symptoms than those who remain unvaccinated. [footnote 55]
The UK’s vaccination programme, which prioritised the most vulnerable to COVID-19 for early receipt of vaccines, has now protected tens of millions of people and prevented many hospitalisations and deaths. [footnote 56] The programme continues to be extended. The NHS has already given a first dose to 60% of 12 to 15 year olds in England and is now offering second doses. [footnote 57] Vaccinations have also started to be offered to at-risk 5 to 11 year olds since week commencing 31 January (2 doses, 8 weeks apart). From April, all 5 to 11 year olds will be able to come forward for a course of COVID-19 vaccine (2 doses, 12 weeks apart). Every parent will have the opportunity to make an informed choice.
The Government will continue to be guided by JCVI advice on the deployment of the vaccination programme. Subject to JCVI advice, further vaccinations (boosters) may be recommended for people who are most vulnerable to serious outcomes from COVID-19 this autumn and, ahead of that, a spring booster for groups JCVI consider to be at particularly high risk.
For people who have yet to take up their initial vaccine offer, the NHS continues to make vaccines available across the UK to ensure that every eligible person who wants a vaccine can get one. The Government will continue to provide flexible delivery models to ensure vaccines remain accessible.
The Government will continue to support communities with lower rates of COVID-19 vaccine uptake, particularly in areas of deprivation and for ethnic minority groups. In December 2021, the Government announced a further £22.5 million in funding for the Community Vaccine Champions Scheme to support 60 local authorities with the lowest COVID-19 vaccine uptake. [footnote 58] Community Champions work with local councils to address barriers to accurate vaccine information and encourage individuals to get vaccinated.
Deploying treatments
The Government has moved quickly since the onset of the pandemic to ensure that those at risk of and suffering from COVID-19 have early access to safe and effective treatments.
In April 2021, the Prime Minister launched the Antivirals Taskforce ( ATF ), in order to identify, procure and deploy novel antiviral treatments for UK patients with COVID-19. Antivirals can be used at the earliest stage of infection to help reduce the development of severe COVID-19 by blocking virus replication.
The ATF has secured a supply of almost 5 million courses of antivirals - more per head than any other country in Europe. [footnote 59] These antivirals are the first medicines which can be given at home to treat people whose immune systems mean they are at higher risk from COVID-19.
In company trials, Paxlovid (nirmatrelvir + ritonavir) reduced the relative risk of COVID-19-associated hospitalisation or death by 88% in unvaccinated patients who received treatment within 5 days of symptoms appearing. [footnote 60] Results from Lagevrio (molnupiravir) company trials show around 30% relative reduction in the rate of hospitalisation in unvaccinated patients. [footnote 61] Both antivirals have now received conditional marketing authorisation from MHRA , making the UK the first country in the world to approve an oral antiviral that can be taken at home for COVID-19. [footnote 62]
People at highest risk of developing severe COVID-19 can now access antivirals should they test positive for COVID-19. UKHSA has sent priority PCR tests to around 1.3 million people to support rapid turnaround of results so they can access the treatments as soon as possible after symptoms begin. [footnote 63] In England, around 14,000 people with weakened immune systems have already been treated with the new antivirals, Lagevrio (molnupiravir) and Paxlovid (nirmatrelvir + ritonavir), and the new monoclonal antibody treatment, Xevudy (sotrovimab).
Therapeutics
The Therapeutics Taskforce was quickly established in April 2020 to ensure that COVID-19 patients in the UK had access to safe and effective treatments as soon as possible. Effective therapeutics have played a vital role in lessening the severity and impact of COVID-19.
The UK has led the way in the testing and deployment of life-saving treatments, which have been made available to patients in the UK and across the world. World-leading clinical trials such as RECOVERY - the world’s largest randomised controlled clinical trial for COVID-19 treatments have helped to discover new effective treatments for COVID-19.
In June 2020, the UK was the first in the world to discover that dexamethasone - a low-cost corticosteroid - reduced the risk of mortality in hospitalised COVID-19 patients requiring oxygen or ventilation by up to 35%. [footnote 64] UK Government-funded trials demonstrated tocilizumab and sarilumab - monoclonal immunomodulatory antibody treatments - reduced the relative risk of mortality by up to 24% when administered to patients within 24 hours of entering intensive care. [footnote 65]
New therapeutics like Xevudy (sotrovimab), a monoclonal antibody, have been authorised for use in people who have mild to moderate COVID-19 infection and at least one risk factor for developing severe illness. In a clinical trial, a single dose of the monoclonal antibody was found to reduce the risk of hospitalisation and death by 79% in high-risk adults with symptomatic COVID-19 infection. [footnote 66]
Supporting the NHS and social care
Throughout the pandemic the Government has provided health and social care services with resources and support to respond to the unique challenges they have faced.
The approach to managing COVID-19 in NHS and adult social care services will continue to evolve in the coming months, but will continue to focus on providing care for those that need it and supporting people who are most vulnerable to COVID-19, including people receiving social care and people receiving treatment in hospitals.
A key objective for the NHS over the last two years has been to keep patients and staff safe and limit the spread of COVID-19 within hospitals. Enhanced Infection Prevention Control ( IPC ) measures have been required in NHS settings, including:
- a. Asymptomatic testing for patients and for staff;
- b. Enhanced personal protective equipment ( PPE ) to protect healthcare workers and the patients they come into contact with;
- c. COVID-19 specific bed management and clinical pathways; and
- d. Evaluation of ventilation in line with the latest guidance. [footnote 67]
In the next phase of managing COVID-19, the NHS will continue to:
- a. Deliver and support specific programmes to manage the risk of COVID-19, including the deployment of vaccines (see chapter 4).
- b. Support patients with Long COVID, where the UK is leading the way in research, treatment and care. Specialist services have been established throughout England for adults, children and young people experiencing long-term effects of COVID-19 infection, underpinned by a £100 million plan for 2021-22, and further investment for 2022-23.
- c. Work to better understand COVID-19 and the long-term health impacts it may have, supported by £50 million in research funding.
- d. Use and develop measures to restore and recover elective services and reduce backlogs for treatments.
- e. Providing access to free PPE to the end of March 2023, or until the IPC guidance on PPE usage for COVID-19 is amended or superseded (whichever is sooner).
Adult social care
Care home residents, and those in receipt of adult social care at home and other care settings, are often among the most vulnerable in society to COVID-19. To protect these people, the Government introduced additional protective measures, including:
- a. Free PPE for adult social care workers;
- b. Prioritisation of staff and residents for vaccinations;
- c. Designated settings to ensure that those who need residential care but are still likely to be infectious with COVID-19 at the point of discharge from hospital can complete a period of isolation before moving to their care home;
- d. Introducing visitor restrictions at times of particularly high risk; and
- e. In recognition of the challenges facing the sector, the Government published its first ever set of winter plans for adult social care.
The Government will continue to support the adult social care sector with the following protections:
- a. Supporting and encouraging the take-up of vaccines amongst care recipients and staff, including any further doses that may be recommended by JCVI for COVID-19 and other infections;
- b. Guidance on precautions for visitors and workers in adult social care; and
- c. Providing access to free PPE to the end of March 2023 or until the UK IPC guidance on PPE usage for COVID-19 is amended or superseded (whichever is sooner).
The role of the Government in managing the COVID-19 response in adult social care has been unprecedented. As a part of living sustainably with COVID-19, by 1 April the Government will publish updated IPC guidance. This will replace current COVID-19 IPC guidance for care homes, home care and other adult social care services. The Government will continue to work with local authorities and care providers to respond to outbreaks in care settings and manage local workforce pressures.
Tackling health inequalities
COVID-19 has also exacerbated pre-existing socio-economic and health inequalities, driving poorer outcomes amongst those who were already disadvantaged. Since the start of the pandemic, the NHS has accelerated its preventative health programmes which proactively engage those at greatest risk of poorer health outcomes to address health inequalities.
The Government will continue to support communities with lower rates of COVID-19 vaccine uptake, particularly in areas of deprivation and for ethnic minority groups as part of its approach to both reducing health disparities as and living with COVID-19, but also to support the wider health and social care system.
The recent ‘Levelling Up the United Kingdom’ white paper also aims to reduce geographical inequalities by investing in health, local infrastructure and leadership, and improving education and skills. [footnote 68] The Government will set out a strategy to tackle the core drivers of inequalities in health outcomes in a new white paper on health disparities in 2022.
The Government has provided significant additional funding to respond to the pandemic on an emergency basis through additional borrowing. As the country moves to living with COVID, the Government must ensure that the cost of resilience and contingency measures are met in a responsible and sustainable manner. The Government is already asking taxpayers to make an additional contribution through the Health and Care levy. The Government will meet the cost of living with COVID-19 within this and other existing funding streams.
5. Maintaining resilience
As set out in the introduction, the future path and severity of the virus is uncertain and it may take several years before the virus becomes more predictable. During this period further resurgences will occur, it is possible more severe variants will emerge and there will sadly be more hospitalisations and deaths. As a result, the Government is taking steps to ensure there are plans in place to maintain resilience against significant resurgences or future variants and remains ready to act if a dangerous variant risks placing unsustainable pressure on the NHS .
The Government’s aim is to manage and respond to these risks through more routine public health interventions. As such, the NHS has developed a range of interventions to respond to COVID-19 demand while protecting NHS activity to the fullest possible extent. In future, pharmaceutical capabilities will be the first line of defence in responding to COVID-19 if risk threatens to place unsustainable pressure on the NHS .
The Government will retain surveillance to monitor the virus, understand its evolution and identify changes in characteristics, enabling the Government to make informed decisions. The Government will prepare and maintain the capabilities to ramp up testing and other tools such as laboratory infrastructure to be used as a line of defence against a new variant.
Monitoring and mitigating risks
The UK has been a global leader in sequencing and monitoring, at times uploading the highest number of sequences of any country on the Global Initiative on Sharing Avian Influenza Data ( GISAID ) platform. [footnote 69] UKHSA will continue to sequence some infections and monitor a range of data.
Domestic surveillance
The Government will continue to monitor cases, in hospital settings in particular, including using genomic sequencing, which will allow some insights into the evolution of the virus. UKHSA will maintain scaled down critical surveillance capabilities including the COVID-19 Infection Survey ( CIS ) population level survey, genomic sequencing and additional data. This will be augmented by continuing the SARS-CoV-2 Immunity & Reinfection Evaluation ( SIREN ) and Vivaldi studies.
UK monitoring mechanisms during the pandemic
The Office for National Statistics ( ONS ) has continued to keep pace with the changing evidence needs of the Government and the public in tracking the spread of COVID-19 and understanding its impact. This includes official statistics on health, society, the labour market and the economy.
The COVID-19 Infection Survey was established in April 2020 to measure:
- How many people across England, Wales, Northern Ireland and Scotland test positive for a COVID-19 infection at a given point in time, regardless of whether they report experiencing symptoms;
- The average number of new positive test cases per week over the course of the study; and
- The number of people who test positive for antibodies.
The results of the survey contribute to UKHSA ’s estimates of the rate of transmission of the infection, often referred to as “R”. The survey provides important information about the socio-demographic characteristics of the people and households who have contracted COVID-19.
The SIREN study was established in June 2020. The purpose of this study is to understand whether prior infection with SARS-CoV2 (the virus that causes COVID-19) protects against future infection with the same virus.
The Vivaldi Study was also established in June 2020. The purpose of this study is to investigate COVID-19 infections in care homes, to find out how many care home staff and residents have been infected with COVID-19, and inform decisions around the best approach to COVID-19 testing in the future.
Preparing to respond
In order to be prepared for further resurgences and new variants, the Government will maintain resilience and infrastructure required to scale up a proportionate response.
NHS and social care resilience
The NHS has developed a range of interventions to respond to COVID-19 demand while protecting urgent and elective care activity to the fullest possible extent, including during the peaks of demand seen in April 2020, January 2021, and at the present time. These interventions include:
- a. Tried and tested plans to expand general and acute and critical care bed capacity as needed, learning the lessons from previous waves of COVID-19. This includes surging capacity within hospital trusts’ existing footprints, across Integrated Care System footprints and clinical networks, and patient transfers between regions if required.
- b. Maximising patient discharge, working with local authorities and partners to ensure that all medically fit patients can be safely discharged as soon as possible, supporting improved patient outcomes and freeing up beds for elective surgery.
- c. Making full use of non-acute beds in the local health and care system as necessary, including in hospices, hotels, community beds and the independent sector. At points throughout the pandemic NHS England has contracted with independent providers to secure additional surge capacity and prevent the NHS from becoming overwhelmed due to COVID-19 infections. The Increasing Capacity Framework streamlines central procurement processes and allows the NHS to effectively secure the capacity it needs to meet patient needs on a local level.
- d. The use of ‘virtual wards’ and ‘hospital at home’ models of care have ensured that patients can be safely cared for in their own homes and that additional bed capacity can be freed up in hospitals. The NHS operational planning guidance sets out that, by December 2023, systems should complete the comprehensive development of virtual wards towards a national ambition of 40 to 50 virtual beds per 100,000 population.
- e. Implementing a range of workforce interventions, including increasing staffing numbers, temporary local adjustments to staffing ratios, with flexible redeployment of staff including training for roles in critical or enhanced care.
- f. Ensuring continued improvements to the urgent and emergency care pathway to avoid emergency department crowding. Interventions include using NHS 111 as the first point of triage for urgent care services, which increases the ability to book patients into the full range of local urgent care services, including urgent treatment centres; same day emergency care; speciality clinics; and urgent community and mental health services.
While significant uncertainty remains, the NHS will continue to closely monitor COVID-19 demand and keep the use of these interventions under review, deploying them as necessary to protect the delivery of health services to the fullest extent possible.
Local authorities will have their own contingency plans for maintaining care services in the event of acute workforce supply challenges. In the event that a local authority – having deployed all its contingency measures – is unable to cope, a request for further support could be made via the Local Resilience Forums ( LRFs ).
The Government will continue to work closely with the health and care sectors to identify and understand capacity risks, in the event of another challenging winter and/or new variant of concern.
Pharmaceutical interventions and medical countermeasures
The Government already has experience in successfully deploying a contingency response based on medical countermeasures. During the response to the Omicron variant, the NHS administered a booster programme to all adults and met the surge in demand for vaccines at short notice. The Government will ensure that there are sufficient procurement plans in place to make certain that the UK has access to the most effective vaccines on the market, and that these are available to the health care system and the public when needed.
Testing: Contingency capabilities
The Government will retain core infrastructure and capabilities in England to scale up testing in the case of a new dangerous variant.
Local outbreak management
Local partners have significantly stepped up to support local outbreak management. In future the Government expects COVID-19 to be managed regionally and locally as part of a wider all hazards approach, using existing health protection frameworks.
The Government will revise current COVID-19 outbreak management advice and frameworks, to set out the support that local authorities and other system partners (such as LRFs , regional health protection teams, the NHS and others) can expect from regional and national stakeholders and the core policy and tools for contingency response. The Government will continue to provide guidance via UKHSA engagement with local partners.
Approach at the borders
Last month the Government announced its new system for international travel, underpinned by a commitment to see a return to unrestricted travel and to support recovery across all sectors. There are now no requirements on eligible vaccinated travellers apart from the need to complete a simplified Passenger Locator Form. Travellers who do not qualify as eligible vaccinated also need to take a pre-departure test and an arrival test on or before day 2, but no longer need to isolate or take a day 8 test.
The Government also committed to developing a contingency toolbox of options. The Government recognises that border measures have carried very high personal, economic, and international costs. The Government will only consider implementing new public health measures at the border in extreme circumstances where it is necessary to protect public health.
Contingency measures would only be used where they are proportionate to the threat faced by a COVID-19 variant and effective in slowing ingress to avert pressure on public services such as the NHS . There may be scenarios where border measures are not appropriate and will not form part of a contingency response. The approach will be underpinned by three important principles:
- a. The bar for implementation of any measures is very high;
- b. Any measure will be tailored and proportionate to the threat posed and will seek to minimise economic and social impacts; and
- c. In the event any measures were deemed necessary they would be time limited and not be in place any longer than needed.
Given the current state of the pandemic and a move towards global travel volumes returning to normal, the infrastructure for hotel quarantine will be fully stood down from the end of March and the Government is developing options to increase compliance with home isolation in its place should quarantine measures need to be reintroduced. Previous global responses to variants of COVID-19 that targeted travel from specific countries may not always be appropriate given how quickly the virus can spread, and tailoring measures to the nature of the threat can improve their effectiveness and proportionality. As such, the Government will have in reserve a more agile toolbox tailored depending on the nature and source of the threat, and deployed only where that high bar is crossed. The default will be to first consider whether less stringent measures are appropriate so as to minimise the impact on general travel where possible.
The Government will set out the contingency approach and toolbox of measures in more detail ahead of Easter when reviewing The Health Protection (Coronavirus, International Travel and Operator Liability) (England) Regulations 2021. The Government will continue to work with industry on contingency planning.
6. Securing innovations and opportunities from the pandemic
The COVID-19 pandemic has been a unique challenge for governments, communities and businesses across the world. These challenges have brought with them opportunities for innovation, as new approaches were developed and deployed at scale and pace. The Government is committed to securing the innovations and opportunities which have emerged during the pandemic, where there is long term benefit to wider Government priorities.
In addition, the COVID-19 public inquiry chaired by Baroness Hallett starts this spring and is intended to enable the Government to learn lessons about its response.
The Government will also remember those that have lost their lives during the COVID-19 pandemic, and commemorate the enormous efforts and sacrifices of all those who have supported the country throughout. On 12 May 2021, the Prime Minister announced that a UK Commission on COVID Commemoration would be established to consider how the country should remember those who have lost their lives and recognise those involved in the response. The Government will set out the Commission’s membership and terms of reference in due course.
Innovation, opportunities and learning
Life sciences.
Over the course of the pandemic the scientific community has made extraordinary scientific advances. The Government directly supported several vaccine manufacturers in their research and development. The first COVID-19 vaccines were ready for clinical trials in under a month, which then led to the deployment of the first safe and effective wide-scale COVID-19 vaccination programme. These vaccines have now been used more widely around the world than many medicines, with extraordinarily successful results.
Innovations in vaccines, antivirals and therapeutics will likely play a vital role in the Government’s response against COVID-19 in the future. A number of vaccine suppliers are already trialling new bi-valent vaccines, which would provide protection against COVID-19 variants. The Vaccine Taskforce ( VTF ) will continue to ensure that the UK has access to effective vaccines on the market. The Therapeutics Taskforce will continue to support the eight national priority clinical trial platforms run by the National Institute for Health Research, focused on prevention, novel treatments, and treatments for Long COVID.
The UK remains an attractive prospect for companies to invest in the UK’s life sciences sector, whether it be as part of an established research and innovation network or in the growing biologics manufacturing industry. At the recent Autumn Budget, the Chancellor announced a further £354 million for UK life sciences manufacturing as part of the Global Britain Investment Fund to support investment into the UK economy. The Vaccine Taskforce’s investment into facilities at UKHSA Porton Down has increased the UK’s capacity to test the efficacy of vaccines against emerging variants.
Vaccine Taskforce ( VTF )
The Vaccine Taskforce was set up in April 2020 to drive forward the development, procurement, and production of a COVID-19 vaccine as quickly as possible, bringing together the Government, academia, industry and international cooperation in science and research. Since then, the VTF has had unprecedented success. Using experience and expertise from the private sector has enabled the UK to build a diverse portfolio of vaccines and secure assured supply through to 2023. This has allowed the NHS to run the largest vaccination campaign in its history.
The Government’s longer-term approach to vaccine procurement will seek to build upon the legacy of innovation from the success of the VTF and look to apply the wider lessons from the past two years to other vaccination programmes.
NHS and social care
The Government will implement lessons learnt from the pandemic in the Health and Social Care sector, drawing on what worked well, and on future clinical advice. In particular, the Health and Social Care Integration white paper sets out the Government’s plans to make integrated health and social care a reality for everyone across England and to level up access, experience, and outcomes across the country. [footnote 70]
Improving NHS data
In March 2020, the NHS COVID-19 Data Cell (a partnership between NHS England and NHS Improvement ( NHSEI ) and NHSX) worked with partners to provide a data analysis and modelling platform that brought together multiple complex data sources from across the health and care system into a single, secure location.
The platform proved invaluable in providing a single version of the truth to support data driven decisions. In a matter of months, this system achieved what would have taken years to develop under non-crisis circumstances.
Virtual Wards
To enable patients to be safely discharged as quickly as possible the NHS established “virtual wards”. This allowed clinicians to use technology to remotely monitor COVID-19 and non-COVID-19 patients while communicating with them at home.
Oximetry@Home
This NHS service provides pulse oximeters to patients with COVID-19 who are at a higher risk, along with supporting information to monitor their oxygen saturation levels at home, with 24/7 access to advice and support. It is usually led by general practice working alongside community teams. The service can help ensure more timely hospital treatment if required.
Emergency registers for health professionals
Section 2 of the Coronavirus Act 2020 has enabled thousands of nurses and other healthcare professionals who no longer work for the NHS to be placed on temporary registers, allowing them to work in NHS services to alleviate workforce pressures during times of emergency.
Following the success of these registers, the Department for Health and Social Care ( DHSC ) will amend legislation to enable the Nursing and Midwifery Council ( NMC ) and the Health and Care Professions Council ( HCPC ) to establish temporary registers to support emergency response arrangements in future.
Strengthening health security at home and abroad
The Government is committed to supporting future health security and resilience.
UK Health Security Agency ( UKHSA )
UKHSA was set up in April 2021 to prepare for, prevent and respond to all hazards to public health. UKHSA has been instrumental in delivering the UK’s response to COVID-19:
- Testing capacity and diagnostics including the largest network of diagnostic testing facilities in British history. The UK has now registered over 467 million COVID-19 tests. [footnote 71]
- Genomic sequencing capabilities where the UK has uploaded over 2 million genome sequences to the international GISAID database, accounting for a quarter of all SARS-CoV-2 genomes shared globally to date. [footnote 72]
- Innovation and technology: the development of the Rosalind Franklin laboratory, and use of innovative new techniques - such as reflex assay technology - strengthened our ability to rapidly detect COVID-19 mutations and support the assessment of variants of concern. At its peak, in December 2021, the Rosalind Franklin Laboratory was processing over 400,000 PCR tests a week. [footnote 73]
UKHSA will continue to lead the wider health protection emergency planning and response system, championing health security across the UK.
International learning and innovation
Epidemics and pandemics are not new, but the rate at which they have occurred has increased during the last 20 years. This increase is thought to be driven by a combination of changes to land use and human behaviours that bring people into closer contact with wild animals, coupled with unprecedented levels of global movement of people and trade.
Supporting global COVID-19 recovery
The UK remains committed to equitable global access to COVID-19 tools to help reduce the risk and frequency of variants of concern, and to contribute to global COVID-19 recovery. The UK has played a leading role in global vaccine access and has committed up to £1.4 billion of UK aid to address the impacts of COVID-19 and to help end the pandemic as quickly as possible. The UK’s commitment included £548 million to support the COVAX Advanced Market Commitment ( AMC ) to deliver COVID-19 vaccines for up to 92 low- and middle-income countries.
The UK’s G7 Presidency delivered a shared commitment to provide one billion doses to vaccinate the world over the next year. As part of this commitment, the Government committed to donate 100 million surplus COVID-19 vaccine doses by June 2022, at least 80% of which will go to COVAX to enable it to further support those in need. The Government exceeded its target of 30 million doses donated by the end of 2021.
Building resilience to global health threats
The Government continues to invest in and develop resilience to global health threats via improved health and biosecurity and pandemic preparedness, examples include:
- a. Biological Security Strategy: Later this year, the Government will publish a refreshed biological security strategy. COVID-19 has reinforced the need for effective preparation for future biological threats to protect the UK against naturally occurring infections, accidental release and potential deliberate misuse by state and non-state actors, in particular through surveillance, risk monitoring and response planning.
- b. The 100 Days Mission and Early Warning Systems: The 100 Days Mission is a global public-private ambition to harness scientific innovation to reduce the impact of future pandemics by making available safe and effective diagnostics, therapeutics, and vaccines within the first 100 days of a future pandemic threat being identified. The Mission was launched as part of the UK’s G7 Presidency in 2021 and the UK is working domestically, with the G7, G20, and international partners to ensure sustainable implementation of the 25 recommendations to ensure the Mission is achieved by 2026.
- c. Pandemic preparedness: The UK is hosting a global pandemic preparedness summit in March 2022, (the Coalition for Epidemic Preparedness Innovations ( CEPI ) Summit) which will explore how the world can better prepare for pandemics by harnessing the power of science to revolutionise how new vaccines can be developed, manufactured, and equitably distributed to end pandemics.
- d. Engagement and reform of the WHO : The UK is supporting work underway to harness the lessons learnt from the COVID-19 pandemic. A stronger architecture for pandemic preparedness and response includes: sustainably financing the WHO ; supporting improvements to the way outbreaks are investigated and the establishment of a Scientific Advisory Group for Origins of Novel Pathogens; and considering amendments to the International Health Regulations (2005) to improve management of public health emergencies.
Improved international consistency on global travel health policies
International travel has been severely disrupted throughout the pandemic, causing difficulties for businesses and passengers. The Government will work further with international partners to discuss how cooperation and alignment of border and travel health policies can be improved. This approach will identify opportunities for standardisation to support global efforts to detect, manage, and respond to new health threats as well as seek to deliver as smooth an experience as possible for passengers, helping to support the recovery of the international travel sector.
7. Legislation
During the pandemic, the Government has had to introduce regulations and legislation involving unprecedented government intervention in order to protect public health, and support individuals, businesses and public services. As part of the implementation of the living with COVID-19 strategy, the Government will make the following legislative changes, subject to appropriate parliamentary scrutiny.
Domestic Restrictions under the Public Health (Control of Disease) Act 1984
The Government has always said that restrictions would not stay in place a day longer than necessary, and is now able to proceed with removing the last domestic restrictions:
- a. The Health Protection (Coronavirus, Restrictions) (England) (No. 3) Regulations 2020 (“No.3 Regulations”) have been in place since 18 July 2020. These powers will be revoked on 24 February. Local authorities will now be required to manage outbreaks through local planning, and pre-existing public health powers, as they would with other infectious diseases.
- b. The Health Protection (Coronavirus, Restrictions) (Self-Isolation) (England) Regulations 2020 have been in place since 28 September 2020, and impose a legal duty on individuals who test positive and certain close contacts to self-isolate. As set out in chapter 3, the legal duty to self-isolate will be lifted on 24 February and be replaced by guidance.
Statutory Sick Pay and Employment and Support Allowance
In light of the Government’s decision to end the legal duty to self-isolate from 24 February, on 24 March:
- a. The Statutory Sick Pay (General) Regulations 1982 and the Statutory Sick Pay (Coronavirus) (Suspension of Waiting Days and General Amendment) Regulations 2020 will be amended to remove COVID-19 provisions. From this date, Statutory Sick Pay (SSP) will no longer be payable from day 1 if people are unable to work because they are sick or self-isolating due to COVID-19. Pre-pandemic SSP rules will apply.
- b. The COVID-19 Employment and Support Allowance provisions within The Employment and Support Allowance and Universal Credit (Coronavirus Disease) Regulations 2020 will automatically expire. From this date, people will no longer be eligible for Employment and Support Allowance because they are self-isolating due to COVID-19. Anyone infected with COVID-19 may, subject to satisfying the conditions of entitlement, still be eligible for Employment and Support Allowance on the basis that they have a health condition or disability that affects their ability to work under the general Employment and Support Allowance regulations.
Vaccines as a Condition of Deployment Regulations
The Health and Social Care Act 2008 (Regulated Activities) (Amendment) (Coronavirus) Regulations 2021 making vaccination a condition of deployment were introduced in Care Quality Commission ( CQC ) registered care homes from 11 November 2021. These regulations require that individuals entering the premises are fully vaccinated, unless otherwise exempt. Regulations to extend vaccination as a condition of deployment to health and wider social care settings were approved by Parliament in December 2021, and its main provisions were set to come into force on 1 April 2022. These regulations would require that anyone providing a CQC regulated activity would also be required to be fully vaccinated, unless otherwise exempt.
After reviewing the latest clinical and scientific evidence, the Government announced its intention to revoke both of the above regulations, subject to consultation and appropriate parliamentary procedure. Whilst vaccination remains the country’s best line of defence against COVID-19, the balance of opportunities and risks of the policy have now changed with the dominance of the Omicron variant. The Government therefore assesses that it is no longer proportionate to require vaccination as a condition of deployment through statute. Professional bodies, the Royal Colleges, the Chief Medical Officer, Chief Nursing Officer and others consider it is a professional responsibility for health and care staff to be vaccinated. The Government has asked the professional regulators to review how this responsibility could be strengthened through their guidance, and will also be consulting on doing so through the Government’s guidance for CQC regulated providers.
A public consultation on revocation concluded on 16 February 2022, and the Government will publish its response shortly. Subject to the outcome of the consultation, the regulations will be revoked ahead of 1 April 2022.
International travel regulations
With the intention to continue to facilitate safe travel and sector recovery, and in the context of having significantly reduced travel restrictions, the Government will review The Health Protection (Coronavirus, International Travel and Operator Liability) (England) Regulations 2021 before Easter and ahead of their expiry date of 16 May.
The Coronavirus Act 2020
The Coronavirus Act 2020 was first introduced in March 2020 and has enabled the Government to support individuals, businesses, and public services during the pandemic.
Temporary provisions
The Government will expire all remaining non-devolved temporary provisions within the Coronavirus Act 2020. Half of the original 40 temporary non-devolved provisions have already expired, as the Government has removed powers throughout the pandemic which were no longer needed. Of the 20 remaining non-devolved temporary provisions, 16 will expire at midnight on 24 March 2022. These are:
- a. Section 2: Emergency registration of nurses and other health and care professionals.
- b. Section 6: Emergency registration of social workers: England and Wales.
- c. Section 14: NHS Continuing Healthcare Assessments: England.
- d. Section 18: Registrations of deaths and still-births.
- e. Section 19: Confirmatory medical certificate not required for cremations: England and Wales.
- f. Section 22: Appointment of temporary Judicial Commissioners.
- g. Section 38: Temporary continuity: education, training and childcare.
- h. Section 39-41: Statutory Sick Pay: funding of employers’ liabilities; power to disapply waiting period limitation; modification of regulation making powers.
- i. Section 45: NHS pension schemes: suspension of restrictions on return to work: England and Wales.
- j. Section 50: Power to suspend port operations.
- k. Section 58: Powers in relation to transportation, storage and disposal of dead bodies.
- l. Section 75 (2) and (3): Disapplication of limit under section 8 of the Industrial Development Act 1982.
- m. Section 81: Residential tenancies in England and Wales: protection from eviction.
- n. Section 82: Business tenancies in England and Wales: protection from forfeiture
The remaining four provisions will be expired within six months. These provisions have enabled innovations in the delivery of public services and the Government is seeking approval to make them permanent through other primary legislation currently before Parliament and due to come into force over the spring and summer. In each case, a final six-month extension is necessary in order to ensure there is no gap in the legislation that enables public service delivery. The relevant provisions are:
- a. Section 30: has supported coronial services throughout the pandemic in England and Wales by enabling inquests, where COVID-19 is suspected as the cause of death, to take place without a jury, helping reduce pressures and backlogs. This provision will be made permanent via the Judicial Review and Courts Bill.
- b. Sections 53 to 55: have allowed thousands of court hearings to take place using audio and video links. Over 12,000 hearings per week have taken place using remote technology across 3,200 virtual courtrooms, helping courts reduce the backlog in cases and bring more people to justice. The provision for remote hearings will be made permanent via the Police, Crime, Sentencing and Courts Bill.
Permanent provisions and devolved governments
There are a number of permanent provisions within the Coronavirus Act 2020 which would require new primary legislation in order to repeal. Some of these provisions are still necessary to support the recovery from the pandemic, including:
- a. Section 11: Indemnity for health service activity: England and Wales. This provision ensures that any gaps in indemnity cover for NHS clinical negligence do not delay or prevent ongoing care. Without this, NHS Resolution would be unable to pay legitimate clinical negligence claims, leaving clinicians exposed to the full cost and patients without compensation.
- b. Section 75(1): Disapplication of limit under section 8 of the Industrial Development Act 1982 ( IDA ). This provision ensures that the financial limits set out in section 8 of the IDA do not hinder the allocation of vital Government schemes for businesses such as the Help to Grow scheme, the Automotive Transformation Fund, and the Offshore Wind Manufacturing Investment Scheme ( OWMIS ).
The Government is committed to removing unnecessary provisions from the statute book as soon as possible and will look for opportunities to do so as the Government’s legislative programme proceeds.
Once the Government has received the conclusions of the COVID-19 public inquiry, it will consider whether further changes to public health legislation are needed. The Public Health (Control of Disease) Act 1984 and any outstanding provisions in the Coronavirus Act 2020 would be in scope for this work.
The Government will also work with the Devolved Administrations, who have used their specific powers within the Coronavirus Act during the pandemic, to help transition provisions into devolved legislation where necessary.
Annex: International comparators
Figure 4: proportion of total population of european countries who have received one dose of covid-19 vaccine [footnote 74].
European countries filtered to the top 30 largest by population
Bar chart showing the percentage coverage of first doses of the vaccine in the top 30 most populous European countries. The UK and Sweden are 11th with 77% of their total populations having received a first dose. Portugal has the highest first dose coverage at 95%.
Figure 5: Date at which 50% of the total population of European countries received their first dose of COVID-19 vaccine [footnote 75]
Chart showing the date when the top 20 most populous European countries, reached 50% first dose vaccination coverage of the total population. The UK is first, achieving this vaccine coverage on 29 April 2021.
The speed of the UK’s initial vaccine rollout in early 2021 had a direct impact on the ability to open up the economy, and ease social restrictions sooner than other comparator countries last summer. The success of the rollout also meant that the UK maintained a lower level of restrictions than most other European comparator countries this winter.
Figure 6: Proportion of total population of European countries that are fully vaccinated with a COVID-19 vaccine [footnote 76]
Bar chart showing the percentage of the total population who are fully vaccinated in the top 30 most populous European countries.The UK is 12th, along with Greece, Netherlands, and Austria, with 72% of their total population having received a full vaccine protocol. Portugal has the highest proportion of their population fully vaccinated at 91%.
Data extracted on 20 January 2022, however, differences in reporting mean dates of underlying data vary by a few days.
Figure 7: Date at which 50% of the total population of European countries were fully vaccinated with a COVID-19 vaccine [footnote 77]
European countries filtered to the top 20 largest by population
Chart showing dates at which the top 30 most populous European countries reached 50% coverage of full vaccinations. The UK is second achieving this on 7 Jul 2021, behind Hungary who reached 50% coverage on 27 Jun 2021. Several countries are yet to meet this milestone.
Vaccine protocols vary by country due to use of different manufacturers. Although the initial protocol (full vaccination) is 2 doses for most vaccines, for a few manufacturers this can be 1 or 3 doses. [footnote 78]
Figure 8: Proportion of total population of European countries who have received a booster dose of COVID-19 vaccine [footnote 79]
Bar chart showing the coverage of booster doses in the top 30 most populous European countries The UK is joint 5th with Germany and Ireland at 56% booster coverage. Denmark has the highest coverage with 62% of their total population having received a booster dose.
Figure 9: Date at which 50% of the total population of European countries received a booster dose of a COVID-19 vaccine [footnote 80]
Chart showing dates at which the top 30 most populous European countries reached 50% booster dose coverage. The UK is first achieving this on 1 January 2022, followed by Denmark who achieved this on 4 January 2022. Several countries are yet to meet this milestone.
Figure 10: COVID-19 tests administered per 1,000 people: G20 countries [footnote 81]
The UK has administered more tests per 1,000 people than any other G20 country since 13 February 2020 (noting that there is no publicly available testing data for China).
Bar chart of tests conducted per 1000 people in the G20. The UK is first, conducting 6,607 tests per 1000 people since 13 February 2020. France is second with 3584 and the US is 4th with 2397.
The methodology for recording daily testing figures varies from country to country. The UK testing figures display the number of PCR and antigen tests conducted across pillars 1 and 2. [footnote 82]
Figure 11: Excess deaths per million in European countries [footnote 83]
Excess deaths are defined as the difference between total deaths during a crisis and the expected number of deaths in ‘normal’ conditions. [footnote 84]
Bar chart showing the number of excess deaths per million in the 30 most populous European countries. The UK is 12th with 1,983 excess deaths per million. Bulgaria has the most excess deaths with 8,627 per million.
Figure 12: Recorded COVID-19 deaths per million in European countries [footnote 85]
Bar chart showing the number of recorded COVID-19 deaths per million for the top 30 most populous European countries. The UK is 14th with 2,355 recorded COVID-19 deaths per million. Bulgaria has the most recorded COVID-19 deaths with 5,075 per million.
This chart shows the number of deaths recorded with COVID-19 on the death certificate. The guidance for including COVID-19 on death certificates varies between countries. It is anticipated that the true number of COVID deaths is higher than the number recorded so excess deaths can provide a more well rounded picture of the impact of the pandemic [footnote 86]
SAGE 105, SAGE meeting on COVID-19 (pdf, 231 KB) , 10 February 2022. ↩
UKHSA , Vaccination in the UK , 18 February 2022. ↩
DHSC , UK COVID-19 vaccines delivery plan (pdf, 714 KB) , 11 January 2021. ↩
Our World in Data, Share of people who received at least one dose of COVID-19 vaccine , 16 February 2022. ↩
Our World in Data, COVID-19 vaccine boosters administered per 100 people , 16 February 2022. ↩
Cabinet Office, COVID-19 Response - Spring 2021 (Roadmap) , 22 February 2021. ↩
Cabinet Office, COVID-19 Response: Autumn and Winter Plan , 14 September 2021. ↩
WHO , Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern , 26 November 2021. ↩
NHSE , Weekly Admissions and Beds 10 February 2022 . ↩
Academics supporting SAGE , Viral Evolution Scenarios , 10 February 2022. ↩
SAGE , Minutes from One-hundred-and-fifth SAGE meeting on COVID-19 (pds, 231 KB) , 10 February 2022. ↩
NERVTAG , Long term evolution of SARS-CoV-2 , 26 July 2021. ↩
Cabinet Office, 100 Days Mission (pdf, 1,114 KB) , 12 June 2021. ↩
The UK does not currently administer the single shot Janssen vaccine. ↩
UKHSA , COVID-19 vaccine surveillance report (pdf, 1,324 KB) , 3 February 2022. ↩
UKHSA ,Vaccination in the UK, 17 February 2022. ↩
UKHSA , Vaccination in England , 17 February 2022. ↩
NHSE , COVID-19 Vaccinations , 17 February 2022. ↩
NHSE , COVID-19 weekly announced vaccinations , 17 February 2022. ↩
Booster uptake for: any black background; Black African; Pakistani; and Black Caribbean adults stands at around 30%, 34%, 34% and 34% respectively in the latest data (to 13 February 2022). UKHSA , National flu and COVID-10 surveillance reports: 2021 to 2022 season, 17 February 2022. ↩
NHSE , Monthly COVID-19 Vaccinations , 10 February 2022. ↩
NHSE , Weekly COVID-19 Vaccinations , 17 February 2022. ↩
ONS , Coronavirus (COVID-19) vaccination uptake in school pupils, England: up to 9 January 2022 , 1 February 2022. ↩
UKHSA , Patients in hospital: Coronavirus (COVID-19) in the UK , 18 February 2022. ↩
ONS , Coronavirus (COVID-19) latest insights: Infections , 18 February 2022. ↩
UKHSA , Healthcare in the UK , 18 February 2022. ↩
UKHSA , Deaths in the UK , 18 February 2022. ↩
There has been a 302% increase in the number of cases waiting over a year to be heard. MoJ and HMCTS , Reducing the backlog in criminal courts (pdf, 458 KB) , 22 October 2021. ↩
DfE , Understanding progress in the 2020/21 academic year: Findings from the summer term and summary of all previous findings (pdf, 1,023 KB) , October 2021. ↩
Office for Health Improvement and Disparities, COVID-19: mental health and wellbeing surveillance report , 18 November 2021. ↩
NHS Digital, Mental Health Services Monthly Statistics, Provisional October, Provisional November 2021 , 27 January 2022. ↩
Demand for the National Domestic Abuse Helpline increased by 22% in the year ending March 2021, compared to the previous year, with the average number of calls and contacts increasing most in the quarters coinciding with the first and third national lockdowns. ONS , Domestic abuse in England and Wales overview , 24 November 2021. ↩
ONS , GDP quarterly national accounts, UK: July to September 2021 , 22 December 2021. ↩
ONS , GDP monthly estimate, UK: December 2021 , 11 February 2022. ↩
Hansard, HC Deb. vol.705 col.1143 , 16 December 2021. ↩
HMRC , Coronavirus Job Retention Scheme statistics , 16 December 2021. HMRC , Self-Employment Income Support Scheme statistics , 16 December 2021. ↩
ONS , GDP monthly estimate, UK, December 2021 , 11 February 2022. ↩
ONS , Homeworking in the UK Labour Market: 2020 , 17 May 2021. ↩
ONS , Earnings and employment from Pay As You Earn Real Time Information, UK , 15 February 2022. ↩
ONS , Vacancies and jobs in the UK , 15 February 2022. ↩
SAGE , Scientific evidence supporting the government response to coronavirus (COVID-19) , 18 February 2022. ↩
Based on modelled risks within Table 3, SAGE EMG paper, Role of Ventilation in Controlling SARS-CoV-2 Transmission (pdf, 1,217 KB) . ↩
SAGE , EMG: Simple summary of ventilation actions to mitigate the risk of COVID-19, 19 October 2020 . ↩
CO2 monitors act as a proxy to measure ventilation. Gov.uk, All schools to receive carbon dioxide monitors , 21 August 2021. ↩
Gov.uk, More support for schools and students as plan B comes to an end , 24 January 2022. ↩
DHSC , Adult Social Care Omicron Support Fund , 10 January 2022. ↩
Hippisley-Cox, J. et al. Risk prediction of COVID-19 related death and hospital admission in adults after COVID-19 vaccination: national prospective cohort study , 17 September 2021. ↩
UKHSA , The effectiveness of vaccination against long COVID (pdf, 443 KB) , February 2022. ↩
The number of hospitalisations directly averted by vaccination. In total, around 261,500 hospitalisations have been prevented in those aged 45 years and over up to 19 September 2021. UKHSA , COVID-19 vaccine surveillance report (pdf, 565 KB) , 21 October 2021. Furthermore, around 105,600 hospitalisations have been prevented in those aged 25 years and over in England from 13 December 2021 to 6 February 2022 inclusive. UKHSA , Boosters prevented over 105,000 hospitalisations, UKHSA analysis estimates , 10 February 2022. ↩
Gov.uk, £22.5m of funding announced in new community push to get nation boosted now , 19 December 2021. ↩
DHSC , Second ground-breaking antiviral to be deployed to country’s most vulnerable , 28 January 2022. ↩
Pfizer, Additional Phase 2/3 Study Results Confirming Robust Efficacy of Novel COVID-19 Oral Antiviral Treatment , 14 December 2021. ↩
Merck, Merck and Ridgeback Announce Publication of Phase 3 Study of Molnupiravir , 16 December 2021. ↩
MHRA , First oral antiviral for COVID-19, Lagevrio (molnupiravir), approved by MHRA , 4 November 2021. ↩
Statement from the Chief Investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial on dexamethasone , 16 June 2020. ↩
Gov.uk, NHS patients to receive life-saving COVID-19 treatments that could cut hospital time by 10 days , 7 January 2021. ↩
Gov.uk, MHRA approves Xevudy (sotrovimab) , 2 December 2021. ↩
NHSE , (HTM 03-01) Specialised ventilation for healthcare buildings , 22 June 2021. ↩
DLUHC , Levelling Up the United Kingdom (pdf, 782 KB) , 2 February 2022. ↩
GISAID Submission Tracking (19 November 2021 - 16 February 2022 the UK uploaded 30% of global sequences). ↩
DHSC , People at the Heart of Care: adult social care reform , 1 December 2021 ↩
UKHSA , Testing in the UK , 17 February 2022. ↩
Gov.uk, UK completes over 2 million SARS-CoV-2 whole genome sequences , 10 February 2022. ↩
UKHSA , Rosalind Franklin laboratory processes 5 million PCR tests , 10 February 2022. ↩
Our World in Data, Share of people vaccinated against COVID-19 . 19 February 2022. ↩
Our World in Data, Share of people vaccinated against COVID-19, 20 February 2022 ↩
Our World in Data, Share of people who completed the initial COVID-19 vaccination protocol, 20 February 2022. ↩
Our World in Data, Coronavirus (COVID-19) Vaccinations , 20 February 2022. ↩
Our World in Data, COVID-19 vaccine boosters administered per 100 people , 20 February 2022. ↩
Our World in Data, Daily new COVID-19 tests per 1,000 people 18 February 2022. ↩
Our World in Data, The Our World in Data COVID-19 Testing dataset . 20 February 2022. ↩
Our World in Data, Excess mortality: Cumulative number of deaths from all causes compared to projection based on previous years, per million people , 20 February 2022. ↩
Our World in Data, Excess mortality during COVID-19 . 20 February 2022. ↩
Our World in Data, Weekly confirmed COVID-19 deaths per million people , 20 February, 2022. ↩
Our World in Data, Coronavirus (COVID-19) Deaths . 20 February 2022. ↩
Is this page useful?
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. Please fill in this survey (opens in a new tab) .
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Medical Devices
- Medical Device Safety
- Safety Communications
At-Home COVID-19 Antigen Tests-Take Steps to Reduce Your Risk of False Negative Results: FDA Safety Communication
November 17, 2022, Update: The FDA took an additional action related to the need for repeat testing following a negative COVID-19 test result on COVID-19 antigen tests -- revising the emergency use authorizations (EUAs) of all authorized COVID-19 antigen tests on November 1, 2022. For details, see FDA Actions below.
Date Issued: August 11, 2022 (Updated November 17, 2022)

The U.S. Food and Drug Administration (FDA) is advising people to perform repeat testing, also called serial testing, following a negative result on any at-home COVID-19 antigen test, to reduce the risk an infection may be missed (false negative result) and to help prevent people from unknowingly spreading the SARS-CoV-2 virus to others. The FDA recommends repeat testing following a negative result whether or not you have COVID-19 symptoms.
At-home COVID-19 antigen tests detect proteins, called antigens, from the SARS-CoV-2, the virus that causes COVID-19. At-home COVID-19 antigen tests are less likely to detect the SARS-CoV-2 virus than molecular tests, such as polymerase chain reaction (PCR) tests. This is especially true early in an infection or in people who do not have COVID-19 symptoms. Currently, all at-home COVID-19 antigen tests are FDA-authorized for repeat use. This means people should use multiple tests over a certain time period, such as 2-3 days, especially when the people using the tests don't have COVID-19 symptoms. Today, the FDA is highlighting the continued need for repeat testing when people get a negative result with an at-home COVID-19 antigen test, including recommending additional testing over a longer period of time.
Over the course of the COVID-19 pandemic, public health scientists have continued to learn about the SARS-CoV-2 virus and the impact of variants on diagnostic tests that detect SARS-CoV-2. Today's recommendations are based on the latest study results from people with likely omicron infection showing that repeat testing after a negative at-home COVID-19 antigen test result increases the chance of an accurate result. COVID-19 diagnostic testing remains a cornerstone of our nation's fight against COVID-19. At-home COVID-19 antigen tests, while not perfect, provide a fast and convenient COVID-19 testing option.
Recommendations:
Before you use a covid-19 antigen test:.
- Be aware that at-home COVID-19 antigen tests are less accurate than molecular tests. COVID-19 antigen tests may not detect the SARS-CoV-2 virus early in an infection, meaning testing soon after you were exposed to someone with COVID-19 could lead to a false-negative result, especially if you don't have symptoms. This is the reason why repeat testing is important.
- If you plan to use at-home COVID-19 antigen tests, have several tests on hand so you can test more than once. You do not need to use the same brand of test each time for repeat testing. Visit At-Home OTC COVID-19 Diagnostic Tests for a list of all FDA-authorized home tests and for more information about who can use a test and for what ages.
- Be aware the FDA expects similar performance with Point of Care (POC) COVID-19 antigen tests performed at a clinic or doctor's office. A negative POC COVID-19 antigen test result should also be followed up with repeat testing and an at-home test could be used.
When you use an at-home COVID-19 antigen test:
Follow the test's step by step instructions exactly to perform the test and to read the test's results.
After you use an at-home COVID-19 antigen test:
- Follow the Centers for Disease Control and Prevention (CDC) guidance for people with COVID-19, including to stay home, isolate from others, and seek follow-up care with a health care provider to determine the next steps.
- If you get a negative result on the second test and you are concerned that you could have COVID-19, you may choose to test again 48 hours after the second test, consider getting a laboratory molecular-based test, or call your health care provider.
- If you get a negative result on the second test, test again 48 hours after the second test.
- If you get a negative result on the third test and you are concerned that you could have COVID-19, you may choose to test again using an antigen test, consider getting a laboratory molecular-based test, or call your health care provider.
- If you get a positive result on any repeat test with an at-home COVID-19 antigen test , you most likely have COVID-19 and should follow the CDC guidance for people with COVID-19.
COVID-19 diagnostic tests detect the SARS-CoV-2 virus. There are at-home COVID-19 diagnostic tests that are FDA-authorized for self-testing at home, or anywhere. The FDA has authorized both molecular and antigen COVID-19 diagnostic tests for home use.
Overall performance of at-home COVID-19 antigen tests
Most at-home COVID-19 tests are antigen tests and do not detect the SARS-CoV-2 virus as well as molecular tests, most of which are laboratory-based such as polymerase chain reaction (PCR) tests. Molecular COVID-19 tests are generally expected to detect the SARS-CoV-2 virus at least 95% of the time when someone is infected. However, at-home COVID-19 antigen tests are generally expected to detect the SARS-CoV-2 virus at least 80% of the time when someone is infected.
When you perform an at-home COVID-19 antigen test, and you get a positive result, the results are usually accurate. However, if you perform an at-home COVID-19 antigen test, you could get a false negative result. This means that the test may not detect the SARS-CoV-2 virus that is in your nasal swab sample. This could happen if you test soon after you get an infection, especially if you don't have COVID-19 symptoms . If you receive a false negative test result, you may unknowingly spread the SARS-CoV-2 virus to others.
Studies to better understand at-home COVID-19 antigen test performance
When at-home COVID-19 antigen tests were initially FDA-authorized, the FDA knew that for people to get accurate results, test instructions would need to include directions for repeat testing. The FDA believed the best way to better understand COVID-19 infections and evaluate test accuracy was to require test developers to perform follow up studies with their tests. The studies would need to assess how well COVID-19 antigen tests could detect the SARS-CoV-2 virus, especially in people without COVID-19 symptoms. Therefore, the FDA required each at-home COVID-19 antigen test manufacturer to assess how well their test works when used by people with and without COVID-19 symptoms following repeat testing instructions.
In parallel, the FDA collaborated with the National Institutes for Health (NIH) and the University of Massachusetts Chan Medical School and together they designed a comprehensive study to assess at-home COVID-19 antigen test performance. The study was funded by the NIH's Rapid Acceleration Diagnostics (RADx) Program and included more than 7,000 participants. The results of the study would be available as a resource to all at-home COVID-19 antigen test manufacturers.
The study participants collected their nasal sample and performed an at-home COVID-19 antigen test. Participants who got a negative test result performed repeat testing every 48 hours, over 14 days. All participants also collected their nasal sample using a home collection kit and then sent the sample to a clinical laboratory for testing with an FDA-authorized molecular test. The study compared the performance of at-home COVID-19 antigen tests to performance of a laboratory-based molecular test. Results from this study show that repeat testing over a longer timeframe improves test performance and increases the likelihood that an at-home COVID-19 antigen test will detect an infection. These results have further guided the FDA's thinking that repeat testing after a negative result with an at-home COVID-19 antigen test reduces the risk of a false negative result.
FDA Actions
On November 1, 2022, based on the data discussed in this safety communication, the FDA revised the authorized uses and required updates to the labeling for all currently authorized COVID-19 antigen tests regarding repeat testing after a negative COVID-19 test result.
For additional information about the EUA revision, visit: Antigen EUA Revisions for Serial (Repeat) Testing
For additional information about at-home tests, visit: At-Home OTC COVID-19 Diagnostic Tests
The FDA is committed to assuring appropriately accurate and reliable at-home COVID-19 diagnostic tests for all Americans and will keep the public informed if significant new information about COVID-19 antigen test performance becomes available.
Reporting Problems with Your Device
If you think you had a problem with your COVID-19 test, the FDA encourages you to report the problem through the MedWatch Voluntary Reporting Form .
Health care personnel employed by facilities that are subject to the FDA's user facility reporting requirements should follow the reporting procedures established by their facilities.
If you have questions, email the Division of Industry and Consumer Education (DICE) at [email protected] or call 800-638-2041 or 301-796-7100.

IMAGES
COMMENTS
You should wear a mask around others for 10 days and test for COVID at least 5 days after your exposure. Stay away from people who may be at higher risk for severe COVID . If you develop symptoms ...
Day 0: This is the day you took the test that was positive or the day you first developed symptoms.; Day 1: This is the first full day following symptom onset or the day you were tested.; Days 0 to 5: Isolate at home and wear a mask when around others.; Day 6: You may end isolation if you had no symptoms (were asymptomatic).If you did not have moderate or severe illness, your symptoms are ...
The updated recommendations align guidance for COVID infection with that for other common respiratory viruses. ... For the five days after you resume your normal activities, you should take extra precautions, like wearing a well-fitting mask and maintaining distance from others, gathering outdoors or in well-ventilated areas, cleaning hands and ...
The answer depends. People are likely the most infectious in the first five days after contracting the virus, health officials state, hence why isolation is recommended. As a precaution, those who ...
Testing before and after travel can lower the risk of spreading the virus that causes COVID-19. If you haven't been vaccinated, the CDC recommends getting a viral test within three days before your trip. Delay travel if you're waiting for test results. Keep a copy of your results with you when you travel. Repeat the test 3 to 5 days after your ...
A: People with COVID-19 could potentially transmit it to others well beyond a day after developing symptoms or testing positive. New guidance from the CDC advises people to isolate until they have ...
Airlines have different rules regarding how soon passengers will be allowed to fly after testing positive for COVID. Certain international airlines insist on a longer delay of up to 14 days ...
Consider getting a COVID-19 test if you: Develop COVID-19 symptoms before, during, or after travel. Will be traveling to visit someone who is at higher risk of getting very sick from COVID-19. Were in a situation with a greater risk of exposure during travel (e.g., in an indoor, crowded space like an airport terminal while not wearing a mask).
Get tested with a viral test 3-5 days after travel AND stay home for 7 days after travel. Even if your test is negative, stay home for the full 7 days. If your test is positive or you have symptoms of COVID-19, isolate yourself to protect others from getting infected and follow public health recommendations If you don't get tested, it's ...
You do NOT need to tested 3-5 days after travel to the United States unless you have symptoms of COVID-19. We know that people can continue to test positive for up to 3 months after they had COVID-19 and not be infectious to others. Can people who have recently recovered from COVID-19 travel? "
Compulsory PCR tests, face masks, vaccination certificates — at the height of the pandemic, travel meant navigating reams of red tape and checking a long list of requirements before you'd even ...
This proof can include your positive COVID-19 viral test result, but it has to be taken no more than 90 days before your flight's departure from a foreign country. Additionally, you will also need a signed letter from a licensed healthcare professional stating that you're cleared to travel back to the U.S.
The Centers for Disease Control and Prevention advises people not to "travel until a full 10 days after your symptoms started or the date your positive test was taken if you had no symptoms ...
The COVID experts we interviewed suggest these pre-trip steps: Pack self-tests and high quality (N95 or KN95) masks. Because you sure don't want to have to hunt them down in an unfamiliar place ...
Those who are not must have a negative test 1-3 days before they travel under CDC guidance. They must either get tested 3-5 days after they return and self-quarantine for 7 days, or self ...
You can leave home after 5 days if you feel fine. You can leave home after 5 days if your fever is gone. Wear a Mask. Wear a mask around other people when you have COVID-19. Wear a mask for 10 days after you start feeling sick. Wear a mask for 10 days after you test positive. Wear a mask with extra protection. The mask must cover your nose and ...
March 1, 2024, 10:01 AM PST. By Erika Edwards. People who test positive for Covid no longer need to isolate for five days, the Centers for Disease Control and Prevention said Friday. The CDC's ...
After 10 days, nobody in the study had infectious virus detectable on a PCR test. And a third study, of 260 vaccinated health care workers in Chicago, found that overall, 43% were testing positive on rapid antigen tests five to 10 days after infection with omicron - even though they felt well enough to return to work.
Incubation periods averaged about five days when the Alpha variant was dominant, about 4.5 days when Beta and Delta were dominant, and about 3.4 days once Omicron took over, according to a 2022 ...
Active COVID-19 infections can feature 20 symptoms or more during the infection, and symptoms may change or come and go throughout the recovery period. One study found that post-acute COVID symptoms ranged anywhere from two weeks to 100 days. It is unclear exactly how long post-COVID syndrome can last.
In 2021, at their peak, there were 2.5 million hospitalizations for Covid-19, and in 2023, that number dropped 60% to 900,000 hospitalizations. The decrease in deaths has been even bigger.
and restart the recommended 5-day isolation period at the time of recurrence of symptoms or a new positive COVID-19 test result. You can end re-isolation after 5 days if you are fever-free for 24 hours without the use of fever-reducing medication and your symptoms are improving. You should also wear a mask for 10 days after rebound.
COVID-19 is the disease caused by the SARS-CoV-2 coronavirus. It usually spreads between people in close contact. COVID-19 vaccines provide strong protection against severe illness and death. Although a person can still get COVID-19 after vaccination, they are more likely to have mild or no symptoms. Anyone can get sick with COVID-19 and become ...
fatigue or weakness. muscle or body aches. new loss of smell or taste. headache. abdominal pain, diarrhea and vomiting. feeling very unwell. If you don't feel well or if you have any symptoms, even if mild, assume you may have COVID-19. Immediately isolate at home and away from others.
According to the CDC, you should be retested if: You tested positive for COVID within 30 days and have COVID symptoms. You tested positive for COVID within 31 to 90 days and have COVID symptoms. You tested positive for COVID within 31 to 90 days and do not have COVID symptoms. Antigen testing is recommended.
CDC released today updated recommendations for how people can protect themselves and their communities from respiratory viruses, including COVID-19. The new guidance brings a unified approach to addressing risks from a range of common respiratory viral illnesses, such as COVID-19, flu, and RSV, which can cause significant health impacts and strain on hospitals and health care workers.
If you have a positive COVID-19 test result, try to stay at home and avoid contact with other people for 5 days after the day you took your test. There is different advice for children and young ...
June 7 (Reuters) - A 15-day course of Pfizer's (PFE.N) COVID-19 antiviral treatment Paxlovid did not relieve symptoms of long COVID, according a study by Stanford University researchers. Currently ...
After 5 days, they may choose to take a Lateral ... Previous global responses to variants of COVID-19 that targeted travel from specific countries may not always be appropriate given how quickly ...
COVID-19 antigen tests may not detect the SARS-CoV-2 virus early in an infection, meaning testing soon after you were exposed to someone with COVID-19 could lead to a false-negative result ...